The Association Between 25 Hydroxyvitamin D and Airway Obstruction in Asthma
K Hutchinson1, C Kerley2, K Bolger3, L Cormican3, Y Rochev4, J Faul3
1Biomnis Ireland, Sandyford, Dublin 18
2BCF Diagnostics, Asthma Research Centre, Dublin 15
3Department of Respiratory Medicine, Connolly Hospital, Blanchardstown, Dublin 15
4National University of Ireland, Galway
Abstract
Since Vitamin D has anti-inflammatory effects we wondered whether the association between low serum 25OHD and airway obstruction in moderate persistent asthma might be explained by inflammatory pathways that worsen asthma. All subjects examined were Irish Caucasians with moderate persistent asthma and none took systemic steroid therapy. In addition to computerized spirometry, we measured BMI, serum 25-hydroxyvitamin D (25OHD), total IgE, Eosinophil Cationic Protein (ECP), and high sensitive C- reactive protein (hs-CRP). One hundred (47 male) subjects completed the testing. Within single level of asthma severity, 25OHD levels were related to post-bronchodilator FEV1/FVC (r = 0.26, p < 0.01), but multiple linear regression analysis demonstrated that the association was not explained by obesity or inflammatory markers. We find a relationship exists between airway obstruction and 25OHD levels in asthmatic adults, and the effect is not explained by the presence of potential confounders such as obesity, allergy and systemic inflammation.
Introduction
A number of studies have demonstrated an association between low serum 25-hydroxyvitamin D (25OHD) concentration and asthma severity1,2, but such links might be explained by lower sun exposure in patients with more severe airway disease. We wondered whether an association exists between low serum 25OHD levels and airway obstruction within a single level of asthma severity. In addition, several inflammatory disorders that are important in human asthma have also been associated with vitamin D deficiency (VDD), including obesity, systemic and allergic inflammation. Serum IgE is closely linked to VDD in children.3,4 In a general population, a relationship exists between VDD and adiposity5 and between 25OHD and hs-CRP.6 We therefore hypothesized that observed associations between serum 25OHD concentrations and airway obstruction might be partly explained by the presence of obesity and other chronic inflammatory processes. In particular we wanted to study patients with a uniform skin phototype because serum 25OHD levels are dependent on it.7 Ireland has a high prevalence of VDD, especially during winter time, due to low levels of sunlight8 (average of 3.5 hours of sunshine each day) and poor dietary intake of oily fish and other vitamin D containing foods.9,10 Since VDD is not generally believed to be more common in asthma, and since replacement is not known to improve lung function in adults, Irish patients with asthma are neither routinely screened nor treated with vitamin D supplements. This paper describes our study of serum 25OHD levels and other serum markers of inflammation in Irish patients with moderate persistent asthma and a uniform skin phototype (Fitzpatrick Skin Phototypes I and II).
Methods
Eligible adult asthmatic subjects (aged =18y) with moderate persistent asthma, who attended the Asthma Clinic in Connolly Hospital Blanchardstown, Dublin, Ireland, were invited to participate in a study of factors contributing to the development of airway obstruction in asthma in Autumn/Winter. All subjects were white Caucasians with Fitzpatrick skin phototype I and II and a diagnosis of asthma for at least 12 months substantiated by a reversibility of forced expiratory volume in 1 sec (FEV1) or by a positive histamine bronchial provocation challenge. All had moderate persistent asthma based on the following criteria: symptoms of recurrent episodes of wheezing, cough, shortness of breath, or a combination of these despite treatment with medium-dose to high-dose inhaled corticosteroids plus long-acting beta agonist (LABA) twice a day; (an Asthma Control Questionnaire (five-question version: ACQ5) of greater than 3.0; at least one asthma exacerbation within 2 years requiring systemic corticosteroid therapy. Skin phototype was determined using the Fitzpatrick classification scale.11 We excluded patients with a recent (less than 1 year) diagnosis of asthma and patients with recent systemic corticosteroid therapy (less than 6 months prior to enrolment); patients who took vitamin D supplements and those with a diagnosis of osteoporosis, liver, renal and celiac disease, or cystic fibrosis; and patients who were current smokers or who took anticonvulsants (or other medications thought to increase the metabolism of vitamin D). All subjects signed an institutional review board-approved written informed consent form and underwent blood draw followed by computerized spirometry. The Connolly Hospital Research Ethics Committee approved the study.
Blood samples were collected, centrifuged, aliquoted, and frozen to -70°C until required. Total serum 25-hydroxyvitamin D level was measured by using competitive chemiluminescence immunoassays (DiaSorin, Dietzenbach, Germany) with inter-assay coefficient of variation (CV) of 6.8%. Total 25OHD is currently considered the best circulating biomarker of vitamin D status as it reflects total vitamin D2 and D3 from dietary and supplemental intake as well as cutaneous inputs resulting from ultraviolet B radiation. 25OHD levels were dichotomized based on the most recent approach of the North American Institute of Medicine 2011 report12-14, which is in line with the positions of the Food Safety Authority of Ireland and the European Food Safety Authority. 25OHD is a measure of risk not diagnostic of deficiency: a level below 30 nmol.l-1 indicates “risk of deficiency”; between 30-50 nmol.l-1 is the range of adequacy for the population. Serum IgE, total calcium, magnesium and hs-CRP were assessed using an Abbott Architect ci8200 analyser: the inter-assay CVs for these assays ranged between 1 and 5.6%. Eosinophil Cationic Protein (ECP) was measured applying ImmunoCAP Technology on the IDM Phadia 250. Spirometry was performed with a computerized spirometer (Jaeger, Masterscreen PFTpro, Würzburg, Germany) and measures were taken 20 minutes after administration of a short-acting bronchodilator (albuterol 80 micrograms).
The best of three measures of FEV1; Forced vital capacity (FVC), and FEV1/FVC values were recorded for analysis. BMI (body mass index) was also calculated and recorded. Statistical methods: We hypothesized that any observed association between 25OHD and airway obstruction might be explained by the presence of confounding variables such as allergy, systemic inflammation, or obesity. We therefore conducted multiple linear regression analysis to address the influence of confounding variables. Since a minimum of 20 subjects is generally required for each variable, we recruited more than 80 subjects to avoid type 1 statistical error (where an effect is not detected due to low sample size). Since 25OHD levels are skewed, we converted values to log scale. We treated each potential confounding variable as a continuous variable by using hs-CRP, IgE and ECP levels, and BMI as measures of systemic inflammation, allergy, and obesity respectively. We employed multiple linear regression analysis and Spearman correlation to determine whether a relationship exists between FEV1/FVC, hs-CRP, BMI, serum IgE, ECP, and log10 serum 25OHD levels. P < 0.05 was considered statistically significant. All analyses were performed using Graphpad prism (Graphpad, San Diego, CA) and Microsoft Excel (Microsoft Inc., Redmond, WA, USA) statistical software.
Results
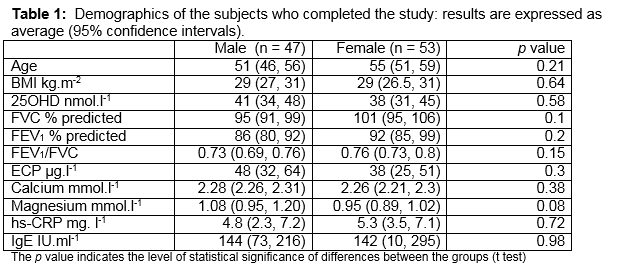
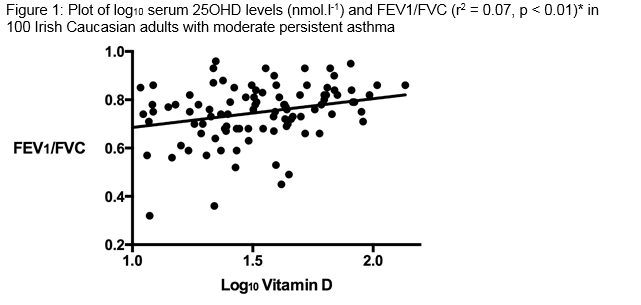
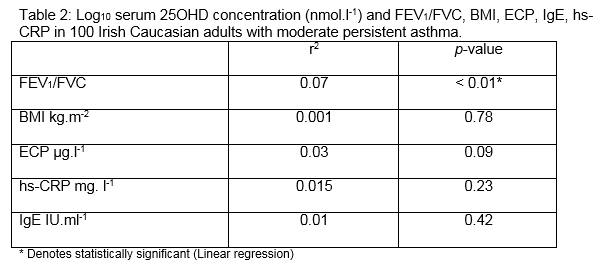
187 subjects with asthma were eligible (from 2500) to enrol for the study because they had skin phototype I and II, moderate persistent asthma, and they had not taken oral corticosteroid therapy within the prior 6 months. A total of 100 patients agreed to participate and all completed the study. The average age was 53 years (95% confidence intervals: 50 to 56 years). Participants were similar in terms of age, BMI and 25OHD levels (Table 1). Approximately one fifth (19%) of subjects were allergic (having a IgE > 200 IU.ml-1 and skin test positivity to one or more of a range of common indoor and outdoor allergens). Almost half (46%) were obese (BMI > 30kg.m-2). The overall level of 25OHD < 30 nmol.l-1 was observed in 45%. The average ± standard deviation (95th percentile) serum 25OHD level was 41 ± 25 (52) nmol.l-1 in men and 38 ± 24 (51) nmol.l-1 in women. There was a significant positive relationship between log10 25OHD concentrations and FEV1/FVC (p < 0.01) (Figure 1). There were no significant relationships between log10 serum 25OHD and ECP, IgE or hs-CRP levels. We performed multiple linear regression using BMI, hs-CRP, ECP and IgE in order to determine whether these variables might influence the strength of our observed association. The observed association between log10 25OHD concentrations and FEV1/FVC remained significant (p = 0.012), but the association was not significantly influenced by any or all of the potential confounding variables of BMI, hs-CRP, ECP, IgE.
Discussion
To our surprise we find that serum 25OHD levels in Caucasian adults with moderate persistent asthma are closely associated with airway obstruction, but this association is not explained by the presence of obesity, or allergic and systemic inflammation. The precise mechanism by which a low 25OHD level might worsen airway obstruction is unclear. In this study, the effect is not closely linked to allergy (as measured by IgE and ECP), although it should be noted that only 20% of our population was atopic. While these data regarding the impact of allergy should be interpreted with caution, it is supported by other studies that suggest the association between VDD and asthma in adults does not appear to be dictated by the presence of allergy. For example, in one large study of the development of adult asthma in Caucasians, VDD was associated with an increased risk of developing asthma, but only in men without allergic rhinitis,15 suggesting some of the pathways involved are not allergy-based and that vitamin D may be linked to non-atopic asthma. We examined whether the presence of obesity explains the link between 25OHD and airway obstruction. Obesity appears to be important in the pathogenesis of asthma and the association between asthma and obesity is stronger in non-atopic than in atopic individuals.16-18 While genetic, developmental, lung mechanical, immunological and behavioural factors have been implicated as playing a causal role between asthma and obesity, recent evidence suggests that asthma associated with obesity can develop through a pathway involving NLRP3, IL-1ß and IL-17 production from pulmonary IL-17+ ILC3 cells.19 IL-17 is an important feature of airway pathophysiology in asthma. Vitamin D treatment significantly inhibits IL-17 production in vitro; thus we expected that an association between airway obstruction and 25OHD might be explained by alterations in inflammatory pathways that are seen in obesity.20 In our sample population the association between 250HD and airway obstruction is not significantly explained by the presence of obesity.
Chronic systemic inflammation (reflected by hs-CRP) is related to VDD 6 and some studies describe reductions in serum hs-CRP after vitamin D supplementation.21 Our observed association between 25OHD levels and airway obstruction is not explained by hs-CRP, although the degree of systemic inflammation in this cohort of patients was low (none had a hs-CRP level greater than 25). It is possible that VDD causes airway obstruction in the presence of high levels of inflammation (during exacerbations of asthma). In earlier studies, 25OHD was also significantly associated with a lower FEV1, a higher BMI, and greater sputum eosinophilia across a range of asthma severities.2 This paper goes further: our data support the idea that VDD affects airway obstruction and that low 25OHD levels might be important in airway biology even within a single level of disease severity. We find that a strong relationship exists between FEV1/ FVC and 25OHD levels in asthmatic adults, and the effect is independent from the presence of potential confounders such as obesity, allergy and systemic inflammation.
Our study does not rule out the possibility of reverse causation: that asthma states may have caused lower 25OHD by virtue of less sun exposure or avoidance of dairy products. A limitation of the study is that there is no estimate of sunlight exposure or a measure of vitamin D intake from diet or supplements. Risk of VDD remains highly prevalent in Irish asthmatic patients, but many studies of Vitamin D supplementation in asthma show very controversial and in some cases detrimental effects on airway obstruction.22,23 In summary, low serum 25OHD levels are positively associated with severity of airway obstruction in Irish Caucasian adults with moderate persistent asthma. The observed association is not explained by differences in asthma severity or skin phototype. The link between airway obstruction and low 25OHD levels in this study of Irish asthmatic adults supports the idea that 25OHD levels might be important for airway obstruction but the effect is independent of some of the commoner inflammatory pathways involved in the pathogenesis of asthma.
Correspondence: K Hutchinson
Biomnis Ireland, Sandyford, Dublin 18
Email: [email protected]
Funding
This study was supported by Research Grants from the Irish Research Council, The Asthma Society of Ireland, and The Irish Thoracic Society.
References
1. Samrah S, Khatib I, Omari M, et al. Vitamin D deficiency and level of asthma control in women from North of Jordan: a case-control study. The Journal of Asthma : Official Journal of the Association for the Care of Asthma. 2014;51:832-8.
2. Korn S, Hubner M, Jung M, Blettner M, Buhl R. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respiratory research. 2013;14:25.
3. Wang SS, Hon KL, Kong AP, et al. Eczema phenotypes are associated with multiple vitamin D pathway genes in Chinese children. Allergy. 2014;69:118-24.
4. Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE - a significant but nonlinear relationship. Allergy. 2009;64:613-20.
5. Pourshahidi LK. Vitamin D and obesity: current perspectives and future directions. The Proceedings of the Nutrition Society. 2014:1-10.
6. Mellenthin L, Wallaschofski H, Grotevendt A, Volzke H, Nauck M, Hannemann A. Association between serum vitamin D concentrations and inflammatory markers in the general adult population. Metabolism: clinical and experimental. 2014;63:1056-62.
7. Malvy DJ, Guinot C, Preziosi P, et al. Relationship between vitamin D status and skin phototype in general adult population. Photochemistry and photobiology. 2000;71:466-9.
8. Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266-81.
9. Cashman KD, Muldowney S, McNulty B, et al. Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. Br J Nutr. 2012:1-9.
10. Cashman KD, Muldowney S, McNulty B, Nugent A, FitzGerald AP, Kiely M, Walton J, Gibney MJ, Flynn A 2013 Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. British Journal of Nutrition 109:1248- 1256
11. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871
12. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA 2011; The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab 96:53-58.
13. Aloia JF 2011 The 2011 Report on Dietary Reference Intake for Vitamin D: Where Do We Go From Here? J Clin Endocrinol Metabol 96:2987-2996.
14. Taylor CL, Thomas PR, Aloia JF, Millard PS, Rosen CJ 2015 Questions About Vitamin D for Primary Care Practice: Input From an NIH Conference. Am J Med 10.1016/j.amjmed.2015.05.025. http://jcem.endojournals.org/cgi/content/abstract/96/1/53
15. Mai XM, Langhammer A, Camargo CA, Jr., Chen Y. Serum 25-hydroxyvitamin D levels and incident asthma in adults: the HUNT Study. American journal of epidemiology. 2012;176:1169-76.
16. Frey U, Latzin P, Usemann J, Maccora J, Zumsteg U, Kriemler S. Asthma and obesity in children: current evidence and potential systems biology approaches. Allergy. 2014.
17. Camargo CA, Jr., Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Archives of internal medicine. 1999;159:2582-8.
18. Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest. 2006;130:890-5.
19. Kim HY, Lee HJ, Chang YJ, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nature medicine. 2014;20:54-61
20. Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in Severe Asthma. Where Do We Stand? American journal of respiratory and critical care medicine. 2014;190:1094-101.
21. Tabesh M, Azadbakht L, Faghihimani E, Tabesh M, Esmaillzadeh A. Calcium-vitamin D co-supplementation influences circulating inflammatory biomarkers and adipocytokines in vitamin D insufficient diabetics: a randomized controlled clinical trial. The Journal of clinical endocrinology and metabolism. 2014:jc20141977.
22. Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. Jama. 2014;311:2083-91.
23. Kerley CP, Elnazir B, Faul J, Cormican L. Vitamin D as an adjunctive therapy in asthma. Part 2: A review of human studies. Pulmonary Pharmacology & Therapeutics 2015; 32: 75-92.
p371