Hospital Acquired Thrombosis (HAT) Prevention in an Acute Hospital; A Single Centre Cross-Sectional Study
MI Khan1, 2, C O’Leary1, A O’Brien2, L Lester1, V Silvari1, C Duggan1, S O’Shea1
1 Haematology Department, Cork University Hospital.
2 Clinical Research Facility, University College Cork.
Abstract
Evidence based guidelines are effective in reducing incidence of venous thromboembolism (VTE) which is associated with morbidly, mortality and economic burden. This study aimed to identify the proportion of inpatients who had a VTE risk assessment (RA) performed and who received thromboprophylaxis (TP), in Cork University Hospital. There was no structured RA tool at the time; information was obtained from medical and drug charts to ascertain if a RA was performed. Patients were then RA by researchers and stratified as per NICE guidelines and the proportion who received TP was calculated. One thousand and nineteen inpatients were screened. Risk was documented in 24% of cases. TP was prescribed in 43.2% of inpatients. Following application of a RA tool >80% were at high risk of VTE with low risk of bleeding with TP prescription in 46.3% of inpatients. A national collaborative effort should be encouraged to develop a standardized approach for safe RA of inpatients and prescription of TP for prevention of HAT.
Introduction
Hospital acquired thrombosis (HAT) is defined as any venous thromboembolic (VTE) event that occurs within 90 days of hospitalisation1,2. VTE includes deep vein thrombosis (DVT) and pulmonary embolism (PE). HAT is associated with significant morbidity, mortality and cost to health systems worldwide3. More than 1.5 million cases of VTE are diagnosed annually in Europe and in excess of 500,000 of these are fatal1. In addition, VTE is the third leading cause of cardiovascular death globally4. For more than three decades, evidence based consensus guidelines for VTE prevention have proven effective and safe5. Despite this evidence, VTE continues to be associated with a major global burden of disease. In 2013, Jha AK (2013); reported 3.9 million cases HAT per annum among 1.1 billion citizens of high income countries6. The use of a mandatory HAT risk assessment (RA) tool and appropriate thromboprophylaxis (TP) in hospitalised individuals significantly reduces VTE incidence1,7. Many countries have introduced mandatory RA tools and TP guidelines. Ireland has been slow to develop such a co-ordinate approach. A national collaborative effort should be encouraged to develop a standardized approach for safe RA of inpatients and prescription of TP for prevention of HAT. This quantitative, cross-sectional study was conducted to determine. The proportion of inpatients who had any VTE risk assessment performed and the number of patients who received TP.
Methods
After ethical approval this study was performed at Cork University Hospital on four designated days between November 2014 and February 2015, each day was two to three weeks apart. Excluding maternity, paediatric, emergency, intensive care and psychiatric patients all adult inpatients above the age of 18 years were included in the study. Obstetric/gynaecology patients were excluded because policies and risk assessment tools have been implemented nationally for this population8. Patients on therapeutic anticoagulation were also excluded. Data was collected by physicians, anticoagulation nurse specialists, and pharmacists. They received training before starting the audit and each of them were provided with a training protocol. Data was collected from inpatients medical and drug prescription charts, and this was recorded on pro-forma sheets. Demographics included age, gender, date of admission, reason for admission, diagnosis and co-morbidities.
In order to ascertain if the patient had a VTE risk assessment on admission or during their hospitalisation, the data collectors were required to obtain the information from the patient’s medical chart as there was no structured risk assessment tool in CUH at the time of the audit. The data collectors were instructed that if there was any reference to the patient’s risk of VTE or bleeding they were to record this as a completed risk assessment. The data collectors were required to review the drug prescription chart to determine if the patient had received TP.
Using the National Institute for Health and Care Excellence (NICE) guidelines for VTE risk assessment and prevention all patients were screened to determine their RA status9.
Following risk assessment patients were stratified into three risk categories as per the NICE guidelines9: high risk of VTE with low risk of bleeding; high risk of VTE with significant risk of bleeding and low risk of VTE. From this the proportion of patients in each group that received TP was calculated.
Results
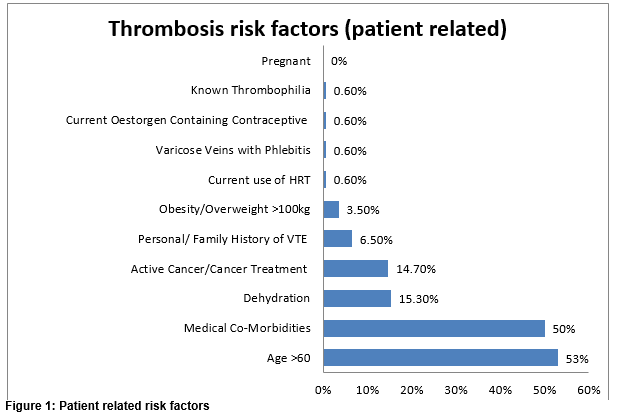
A total of 1,019 patients were screened in the study. The majority of these patients were medical 63.5% (n=648) and 36.4% (n=371) were surgical. The mean age of patients was 69 years. Females accounted for 52% (n=530) of patients. Average length of hospitalisation for each patient at the time of the audit was 6 days (range 1-664 days). There was no formal RA tool in place at the time; researchers recorded any written evidence of RA in patients’ medical chart as RA completed. VTE risk assessment was recorded in 24% (244/1019) of all charts reviewed. TP was prescribed in 43.2% (441/1019) of patients. Patient related risk factors for VTE in the high risk group are shown in figure 1. There were one or more risk factors in each patient.
Risk stratification showed that more than 80% of patients were at high risk of VTE with a low risk of bleeding (Table 1).
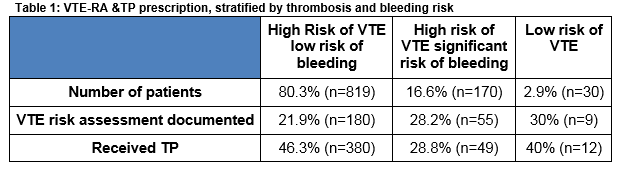
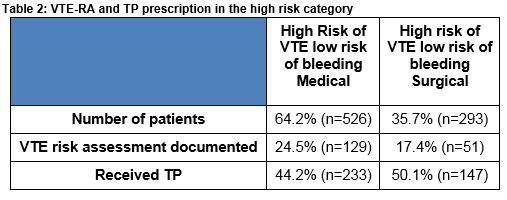
Within the high risk category, 35.7% (n=293/819) of patients were surgical and 64.2% (n=526/819) were medical. TP was administered to 46.3% (380/819) of patients in the high risk category. This was almost evenly distributed between surgical 50.1% (n=147/293) and medical 44.2% (n=233/526) patients (Table 2).
Discussion
This cross-sectional study demonstrates an under-utilisation of safe, effective and recommended means of VTE prevention. Only a quarter of admissions had a VTE–RA recorded in the medical notes. This could be due to the lack of a standardised system for formal RA in the admission process or patients' ongoing care plans. In addition, there may be a lack of awareness of the importance of RA and available tools for VTE prevention amongst physicians. Physicians may not be aware of the high rates of morbidity and mortality associated with HAT, and they may be over estimating bleeding risk. The NICE guidelines for reducing VTE risk recommend the use of the UK National VTE Risk Assessment tool for all patients on admission10. The RA tool not only correctly identifies high risk patients, but also identifies those at low risk who do not require TP. Administering TP to those at low risk of VTE exposes patients to unnecessary risk.
More than 80% of patients in this study were at high risk of VTE with low risk of bleeding. Results from this study are comparable to a national audit carried out by Adamali, H. in 2013 which reported that 90% of patients were within the high risk category for VTE11. The ENDORSE study, which included a greater number of patients, reported that 51.8% were in the high risk VTE category12. This difference may be due to the fact that age greater than 60 years was not considered a separate risk factor for thrombosis in the ENDORSE study. Since the ENDORSE study, age greater than 60 years has been identified as an independent risk factor for VTE13,14. Age was included as a risk factor in this study as per NICE 2010 guidelines9 in which 53% of those in the high risk group were older than 60 years. In Ireland, recent census data reveal that more than 33.2% of the hospitalised population are older than 65 years (excluding maternity)15. This could also explain the increased number of patients in the high risk category in our results.
This study highlights that medical inpatients are at higher risk of VTE compared to surgical inpatients. The ENDORSE study findings report the reverse11. This may reflect the higher proportion of medical patients captured in our data, their complexity and advanced age.
Overall, 46% of high risk patients received TP. A slightly higher percentage of surgical patients received TP compared to medical patients. These results are comparable to other studies12,16,17. The reason for under utilisation of VTE prophylaxis in medical patients may be a more heterogeneous group in terms of underlying disease, frailty and mobility status as compared to surgical patients, and this is due to the combination of risk factors (age, heart failure etc.)18. The importance of TP for the prevention of VTE is widely accepted in surgical patients’ care and is often driven by the type of surgery they undergo. Our slightly lower percentage of surgical patients receiving TP, compared to the ENDORSE study, may be due to the fact that elective orthopaedics is not located at this hospital. This hospital’s orthopaedic department deals with complex trauma only. As the trauma population have a generally higher risk of bleeding, RA and timing of TP needs careful consideration.
The preliminary results of this study were available in March 2015. The results led to the establishment of a Hospital Thrombosis Group (HTG) which developed a user friendly VTE risk assessment (RA) tool and TP policy. The VTE RA tool was incorporated into the hospital’s drug prescription chart which also included a pre printed prescription for a prophylactic dose of low molecular weight heparin (LMWH). A VTE risk assessment is now mandatory in Cork University Hospital, and TP needs to be prescribed, if appropriate, within 24hrs of admission. These new measures were successfully piloted over a four week period in the hospital’s acute medical assessment unit before role out to the entire adult hospital population. This study will be repeated one year post introduction of this initiative, with a target of greater than 90% compliance.
One of the limitations of this study is that the hospital reviewed is a tertiary referral centre and its TP practices may not reflect national practices. Secondly, certain populations such as orthopaedics have been under represented. Finally, as there was no formal RA tool employed at the time of the study, the data was extracted from notes written into the medical chart therefore the completion of RAs may have been underestimated.
A risk assessment tool not only correctly identifies those at high risk of HAT but also, importantly, identifies those at low risk who do not require TP. The current figure of less than 50% prescription of TP for those at high risk of HAT is a major safety concern. Immediate steps were taken locally to introduce a RA tool and TP policy as per international expert guidelines. National implementation of guidelines may have important implications for patient care, safety and hospital budgets, as shown in other countries that have adopted standardized use of RA and TP policy. A co-ordinated national approach is required to implement and ensure compliance with guidelines. Regular audit of practices and dedicated professionals such as venous thromboembolic event (VTE) prevention nurses or thromboprophylaxis officers will help guide change. These roles should involve root cause analyses of all cases of VTE within 90 days of hospitalisation to ascertain if they are related to hospitalisation. Findings should be presented to the disciplines associated with proven cases of VTE so that reflection, learning and necessary practice changes can occur. An e-learning module for HAT prevention could be an important requirement, for all consultants, non-consultant hospital doctors, nursing staff and pharmacists to help raise awareness of the risks and prevention of HAT. In Ireland, a national collaborative approach should be encouraged to develop and implement a standardized, safe RA of inpatients and prescription of TP which is essential for prevention of HAT.
Acknowledgements
The authors thank the Irish Haemostasis Research Foundation Limited for providing an educational grant to Dr. Khan to complete his MD. We thank the Clinical Research Facility UCC for facilitating Dr. Khan’s research fellowship. A special thanks to all data collectors whose work was invaluable and this research would not have been possible without their help.
Conflicts of Interest
The authors declare no conflict of interest.
Correspondence: Aisling O’Brien, Haematology Department, Cork University Hospital
Email: [email protected]
References
1) Cohen AT; Agnelli G; Anderson, FA; Arcelus, JI; Berggvist, D; Brecht, JG; Greer, IA; Heit, JA, Hutchinson, JL; Kakkar, AK; Mottier, D; Oger, E; Samama, MM; Spannagl, M. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756-64
2) Sweetland, S; Green, J; Liu, B; Berrington de González; Canonico, M; Reeves, G; Beral, V. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 2009;339:b4583.
3) House of common health committee. The prevention of venous thromboembolism in hospitalised patients. 2005.London, Department of Health
4) Goldhaber, SZ: Pulmonary embolism thrombolysis: A clarion call for international collaboration. J Am Coll Cardiol 1992;19(2):246-247
5) Clagett, GP; Anderson Jr, FA, Levine, MN; Salzman, EW; Wheeler, HB. Prevention of venous thromboembolism. Chest 1992; 102 (4 Suppl.): 391S-407S.
6) Jha, AK; Larizgoitia I; Audera-Lopez, C; Prasopa-Plaizier, N; Waters, H; Bates, DW. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf 2013; 22: 809-15
7) Rashid, ST; Thursz, MR; Razvi, NA; Voller, R; Orchard, T; Rashid, ST; Shlebak, AA. Venous thromboprophylaxis in UK medical inpatients. J R Soc Med 2005;11:507–12
8) Institute of Obstetricians and Gynaecologists; Royal College of Pysicians of Ireland; HSE Clinical Care Programme in Obstetrics and Gynaecology; Irish Haematology Society. ClinicalPractice Guideline Venous Thromboprophylaxis in Pregnancy.2016; 20: 1-28
9) Venous Thromboembolism: reducing the risk. NICE clinical guideline 92; January 2010.
10) Hunt, BJ ; the prevention of hospital acquired venous thromboembolism in the United Kingdom. Br J Haematol 2009 Mar 144(5):642-52.
11) Adamali, H; Suliman, AM; Zaid, H; Suliman, AM; O’Donoghue, E; Burke, A; Suliman, AW; Salem, M; O’Toole, A; Ibrahim Yearoo, A; Javid, S; Ullah, I; Bolger, K; Dunican, E; McCullagh, B; Curtin, D; Lonergan, MT; Dillon, L; Murphy, AW; Gaine, S . a national house staff audit of medical prophylaxis in medical patients for the PREVENTion of Venous ThromboEmbolism (PREVENT-VTE). IMJ 2013;106(10):302-305
12) Cohen, AT; Tapson, VF; Bergmann, JF; Goldhaber, SZ; Kakkar, AK; Deslandes, B; Huang, W; Zayaruzny, M; Emery, L; Anderson, FA Jr. Venous Thromboembolism risk and prophylaxis in the acute hostial care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008; 371-387-94
13) Spyropoulos, AC; Anderson, FA Jr; Fitzgerald, G; Decousus, H; Pini, M; Chong, BH; Zotz, RB; Bergmann, JF; Tapson, V; Froehlich, JB; Monreal, M; Merli, GJ; Pavanello, R; Turpie, AG; Nakamura, M; Pivovella, F; Kakkar, AK; Spencer, FA. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706-714.
14) Alikhan, R; Cohen, AT; Combe, S; Samama, MM; Desjardins, L; Eldor, A; Janbon, C; Leizorovicz, A; Olsson, CG; Turpie, AG. Risk factors for VTE in hospitalised patients with acute medical illness: analysis of the MEDINOX study. Arch Intern Med 2004;164(9):963-8
15) Health Research and Information Division (2013). Activity in Acute Public Hospitals in Ireland. AnnualReport 2012. Dublin:ESRI
16) Tapson, VF; Decousus, H; Pini, M; Chong, BH; Froehlich, JB, Monreal, M; Spyropoulos, AC; Merli, GJ; Zotz, RB; Bergmann, JF; Pavanello, R; Turpie, AG; Nakamura, M; Piovella, F; Kakkar, AK; Spencer, FA; Fitzgerald, G; Anderson, FA; Anderson, FA Jr. Venous thromboembolism prophylaxis in acutely ill hospitalised medical patients: findings from the international medical prevention registry on venous thromboembolism. Chest 2007;132:936-45
17) Kahn, SR; Panju, A; Geerts, W; Pineo, GF; Desjardins, L; Turpie, AG; Glezer, S; Thabane, L; Sebaldt, RJ. Multicentre evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Throm Res 2007; 119:145-55.
18) Leizoro, A Leizoroovicz, A; Mismetti, P; Preventing venous thromboembolism in medical patients. Circulation 2004;110(4)1: p13-9.
(P547)