Post-Chemotherapy Retroperitoneal Lymph Node Dissection in Patients with Non-Seminomatous Germ Cell Tumour (NSGCT)
Nur A Ariffin1,2, Gregory J Nason1, Shawgi I Omer1, Shane W Considine1, Paul Sweeney1, Derek G Power2
1Department of Urology, Mercy University Hospital, Cork, Ireland
2Department of Medical Oncology, Mercy University Hospital, Cork, Ireland
Abstract
Introduction
Metastases from testicular cancer commonly follows a predictable path via lymphatic spread to the retroperitoneal lymph nodes. The aim of this study was to assess the utilisation and outcomes of post chemotherapy retroperitoneal lymph node dissection (PC-RPLND).
Methods
A retrospective observational study was performed of a prospectively maintained testes cancer database.
Results
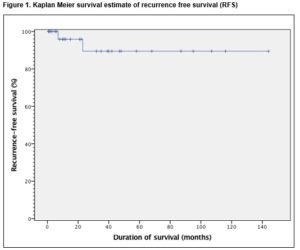
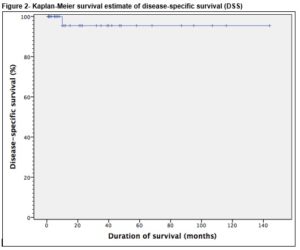
Thirty six patients underwent a PC-RPLND. The final histopathology demonstrated teratoma in 26 (72.2%) patients, viable cancer in 4 (11.1%) patients and necrotic tissue in 6 (16.7%) patients. There was a 55.6% (n=19) postoperative morbidity rate, 78.9% (n=15) in the early (30-day) postoperative period and 21.1% (n=4) in late postoperative period. The mean follow-up of patients was 35 months (range 1-144). Overall, the 5-year recurrence free survival and disease specific survival were 94.4% and 97.2%, respectively.
Conclusion
The peri-operative management and overall outcomes of PC-RPLND in our series are in-line with international standards.
Introduction
Testicular cancer is the most common neoplasm in 20- to 35-year-old men and accounts for one percent of all male neoplasms1,2. It has an incidence of 6.2 per 100,000 per year and an overall mortality of 0.3 deaths per 100,000 per year in the Irish male population3. It is also one of the most curable malignant conditions and has become a model for the multimodal management of malignancies4.
Metastases from testicular cancer follow a predictable path via lymphatic spread to the retroperitoneal lymph nodes5. The primary treatment of nodal disease is platinum-based chemotherapy. Twenty to fifty percent of patients have metastatic disease after chemotherapy, in the form of significant residual retroperitoneal disease. In this subset of patients, surgical resection of the residual disease burden plays an important part6.
In order to achieve complete cure, it is essential for these persistent lymph nodes to be resected as they may contain mature teratoma in 30-40% of patients or viable cancer in 10-20% of patients7. Teratoma is relatively resistant to chemotherapy and has a malignant transformation potential to equally chemotherapy resistant disease. Thus, resection of teratoma is imperative1. In patients presenting with disease in the retroperitoneum as the only location of metastasis after chemotherapy, post chemotherapy retroperitoneal lymph node dissection (PC-RPLND) has the potential to cure almost 80% of cases8.
Recently, Considine et al., in an update of only other RPLND series in Ireland reported low recurrence rates following PC-RPLND9. Complex surgical procedures such as PC-RPLND which have accepted complication rates need to be centralised to high volume units10. The aim of this study was to assess the utilisation and outcomes of PC-RPLND.
Methods
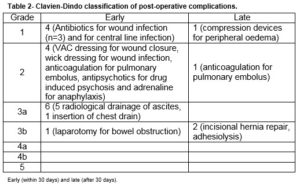
A retrospective observational study was performed of a prospectively maintained testes cancer database. All patients who underwent a PC-RPLND at a tertiary referral university teaching hospital between 2002 and 2015 were included. All procedures were carried out by a single surgeon who had completed a dedicated urological oncology fellowship with a specific interest in testicular cancer. Clinical demographics, histopathological characteristics and peri-operative details were obtained from patient charts and the prospective database maintained by the departmental cancer clinical nurse specialist. Post-operative complications were described by severity according to the Clavien-Dindo classification of surgical morbidities11. Early postoperative morbidity was defined as any adverse event that arises within 30 days of the surgery that altered the typical course of recovery, and late morbidity was any event occurring beyond the 30-day period.
In order to analyse recurrence free survival (RFS), recurrence was noted as an event and defined as radiological or pathological recurrence of viable cancer. RFS was calculated from the date of PC-RPLND to the date of recurrence. In order to analyse disease specific survival (DSS), death from disease was noted as an event, and patients who survived were censored at their last date of follow-up. DSS was calculated from the date of PC-RPLND to the date of disease-specific death. In this study, death from disease was defined as death from testicular cancer or its treatment. Patients who died of other causes (if any), were censored at their date of death. Statistical analysis was performed using Kaplan-Meier survival curves with SPSS version 20 (Armonk, NY, USA). The proposed study received ethical approval from the Clinical Research Ethics Committee (CREC) of the university teaching hospital.
Results
During the study period, 36 patients underwent a PC-RPLND for metastatic non-seminoma germ cell tumour (NSGCT). The final histopathology demonstrated teratoma in 26 (72.2%) patients, viable cancer in four (11.1%) patients and necrotic tissue in 6 (16.7%) patient, Table 1.
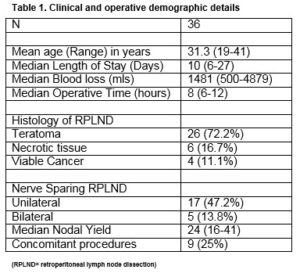
All patients underwent a standard bilateral template RPLND. Nerve sparing was performed in 61% (n=22) of patients (13.8% (n=5) bilateral, 47.2% (n=17) unilateral). The median operative time was 8 hours (range 6-12) and the median estimated blood loss was 1,481 mL (range 500-4879). The median length of hospital stay was 10 days (range 6-27). The median nodal yield was 26 (range 16-41). Nine concomitant procedures- nephrectomy (n=2), orchidectomy (n=3), duodenal resection (n=2), appendicectomy (n=1), inferior vena caval (IVC) resection (1) were performed.
There was a 55.6% (n=19) postoperative morbidity rate, Table 2. 78.9% (n=15) in the early (30-day) postoperative period and 21.1% (n=4) in late postoperative period. Early morbidity (n=14) included ascites (n=5), wound infection (n=3), pneumothorax (n=1), delayed wound closure (n=1), drug-induced psychosis (n=1), bowel obstruction (n=1) requiring laparotomy, pulmonary embolism (n=1), central line infection (n=1) and an anaphylactic reaction (n=1). The five patients with ascites required radiological assisted peritoneal drainage. Drains were not routinely used post-operatively. The three patients with wound infection were treated with intravenous antibiotics and one of them had vacuum assisted drainage device applied due to a superficial wound dehiscence. One patient developed an anaphylactic reaction intra-operatively and on day 5 post-operatively- the causative agent was deemed to be gelatin.
Late morbidity (n=4) involved peripheral oedema (n=1) managed by customised compression device, adhesions (n=1), pulmonary embolism (n=1) and an incisional hernia (n=1). There was no perioperative mortality involved in this study. The mean follow-up of patients was 35 months (range 1-144). Thirty-four (94.4%) remain in complete remission while 5.6% (n=2) had disease recurrence. Of these, only one recurred within the previous dissection field (around the site of prior cavotomy), 23 months after PC-RPLND. This patient was subsequently managed by surgical excision of a portion of the IVC and the recurrent tumour within it, and tubular graft replacement. The other patient had radiological relapse within 7 months post surgery, outside the previous dissection field (around the retrocrural nodes and adjacent spleen). This patient subsequently had progressive disease in the mediastinum and neck which led to a combined surgery of neck, thorax and upper limb girdle. One patient was died of progressive disease following unresectable residual disease 10 months post PC-RPLND. Clearance was not achieved at first setting due to encasement of duodenal and renal vessels, and he subsequently developed retroperitoneal, liver and mediastinal residual disease. These persisted despite additional aggressive chemotherapy post surgery. Overall, the 5-year RFS and DSS were 94.4% and 97.2%, respectively, Figure 1 and 2.
Discussion
The incidence of testicular cancer in Ireland is 6.2 per 100,000 per year (132 new cases per year)2 and the lifetime risk of dying from this cancer is about one in 5,0003. It predominantly affects young men and most of these never require addition surgical intervention, e.g. RPLND. As a result, the overall number of RPLNDs performed annually is low. In the US, RPLND is a standard approach for the surgical management of non-seminomatous germ cell tumors in both the primary and post-chemotherapy setting. The total lymph node yield of the RPLND is an independent predictor of disease recurrence, with more lymph nodes removed associated with improved outcomes. As is the case with other complex surgeries, RPLND performed by higher volume surgeons is associated with higher total lymph node count and may therefore be associated with improved outcomes and long-term survival12. Despite this, recent literature has shown that the median number of RPLNDs logged annually was one, with three urologists performing 23% of all RPLNDs performed in the United States. Seventy-five percent of urologists logged a single RPLND while urologists who logged two RPLNDs in a year were in the top 25% of performers with over half (52%) of all RPLNDs performed by urologists who logged one or two RPLND10. A recent national review of RPLND performed in the UK demonstrated 162 men underwent RPLND by 20 surgeons in 17 centres. The mean (range) case volume per centre was 9 (2-32) and the median (range) case volume per surgeon was 6 (1-30)13. In comparison Ireland- there are only a few surgeons (n=3) undertaking this surgery however our volume compares favorably. We report the experience of a single surgeon performing RPLND in the post chemotherapy setting.
The histological finding of RPLND specimens varies- with documented residual teratoma up to 60%6,8,14,15. Teratoma and necrotic tissue are the most common histology found with viable cancer rates at ~10%16. In a review by Heidenreich, teratoma was found in 30-40% of patients, viable germ cell tumour (GCT) in 10-20% and fibrosis found in remaining selection of patients17. Residual teratoma has the capacity to grow and invade local structures, leading to growing teratoma syndrome18. Malignant transformation has also been observed19. Late recurrence is also a risk for patients with teratoma, which increases the potential of being refractory to chemotherapy20. Viable cancer and teratoma was present in 83% of our series. Following RPNLD, only one patient in our cohort developed a recurrence within the field of dissection. The complexity of the surgery is highlighted by the number of additional procedures (22%) included such as nephrectomy and vascular resections. Djaladat et al. demonstrated that Thirty percent of patients require adjuvant procedures at the time of post-chemotherapy RPLND6. In that series 15% of adjuvant procedures were vascular such as aortic or caval resection/replacement while nephrectomy comprised 14%. Considine et al., reported a rate (28%) of additional procedures similar to our series however a much higher concomitant nephrectomy rate of 20.5% (n=16)9. The template of dissection and the disease burden has an impact on this.
Furthermore, there is considerable morbidity associated with the extensive resection however the peri-operative mortality rate is low21- possibly due to the fitness of these young male patients- there were no peri-operative deaths in our series. There was no peri-operative mortality in our cohort. We did experience an early complication rate of 42% however this reduced to 19% when minor (Clavien Dindo grade 1 and 2) complications were excluded. These findings are in line with a review of primary and PC-RPLND complications by Baniel et al22. It is acknowledged that PC-RPLND is a greater surgical challenge than primary RPLND and has an expected higher morbidity rate. The ability to perform a nerve sparing RPLND adds an additional layer of complexity to the procedure however can reduce the significant side effect of retrograde ejaculation which is a major factor for such young men. Despite this, our series demonstrates favourable recurrence free survival and disease specific survival rates.
Our study is only the second series of RPLND reported in Ireland. Metastatic testicular cancer is a rare disease and the number of PC-RPLND performed is low. Our retrospective series is limited in numbers but presents the single surgeon experience over 14 years with favourable peri-operative outcomes and follow up. Complex surgery such as RPLND needs to be performed by high volume surgeons- in Ireland currently there are only three surgeons performing this procedure in three separate centres. There are ongoing discussions regarding the centralisation of this service. In this study, we report a single centre experience of PC-RPLND for metastatic NSGT of the testes. The peri-operative management and overall outcomes are in-line with international standards- due to the small caseload and complexity of this disease we advocate the management of these cases in a limited number of tertiary referral centres.
Acknowledgements: None
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
No conflict of interest to declare
Ethical Approval:
The proposed study received ethical approval from the Clinical Research Ethics Committee (CREC) of the university teaching hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Correspondence:
Mr Gregory Nason, Specialist Registrar in Urology, Department of Urology, Mercy University Hospital, Cork, Ireland
Email: [email protected]
Phone: +35342198764
References
1. Carver BS, Sheinfeld J. Postchemotherapy surgery for germ cell tumors of the testis. Curr Opin Oncol. 2011 May;23(3):271-4
2. Casey RG, Aktar M, Hegarty P, Butler M, Thornhill JA. A prospective 10 year audit of a single Irish centre’s experience of retroperitoneal lymph node dissection for metastatic testis cancer. Surgeon. 2008 Oct;6(5):294-6.
3. The National Cancer Registry Ireland (2015) Testis cancer factsheet. http://www.ncri.ie/sites/ncri/files/factsheets/FACTSHEET_testis_1.pdf.2015
4. El Sayed S, Grando JP, Almeida SH, Mortati Neto N, Moreira HA. Post-chemotherapy residual mass in non-seminomatous testicular cancer. The role of retroperitoneal lymph node dissection. Int Braz J Urol. 2004 Sep-Oct;30(5):384-8.
5. Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, Nicolai N, Oldenburg J; European Association of Urology..Guidelines on testicular cancer: 2015 update. Eur Urol. 2015 Dec;68(6):1054-68
6. Djaladat H, Nichols C, Daneshmand S. Adjuvant surgery in testicular cancer patients undergoing postchemotherapy retroperitoneal lymph node dissection. Ann Surg Oncol. 2012 Jul;19(7):2388-93.
7. Heidenreich A, Pfister D, Witthuhn R, Thüer D, Albers P. Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection. Eur Urol. 2009 Jan;55(1):217-24.
8. Luz MA, Kotb AF, Aldousari S, Brimo F, Tanguay S, Kassouf W, Aprikian AG. Retroperitoneal lymph node dissection for residual masses after chemotherapy in nonseminomatous germ cell testicular tumor. World J Surg Oncol. 2010 Nov 9;8:97.
9. Considine S, Heaney R, Conroy R, Thornhill JA. Post-chemotherapy retroperitoneal lymph node dissection in the management of metastatic testis cancer: the 16-year experience in an Irish setting. Ir J Med Sci. 2015 Dec 21.
10. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five year experience. Ann Surg. 2009 Aug;250(2):187-96
11. Thompson RH, Carver BS, Bosl GJ, Bajorin D, Motzer R, Feldman DR, Reuter VE, Sheinfeld J. Contemporary lymph node counts during primary retroperitoneal lymph node dissection. Urology. 2011 Feb;77(2):368-72
12. Flum AS, Bachrach L, Jovanovic BD, Helenowski IB, Flury SC, Meeks JJ. Patterns of performance of retroperitoneal lymph node dissections by American urologists: most retroperitoneal lymph node dissections in the US are performed by low-volume surgeons. Urology. 2014 Dec;84(6):1325-8
13. Wells H, Hayes MC, O'Brien T, Fowler S. Contemporary retroperitoneal lymph node dissection (RPLND) for testis cancer in the UK - a national study. BJU Int. 2016 Jun 29. doi: 10.1111/bju.13569. [Epub ahead of print]
14. Beck SD, Foster RS, Bihrle R, Donohue JP, Einhorn LH. Is full bilateral retroperitoneal lymph node dissection always necessary for postchemotherapy residual tumor? Cancer. 2007 Sep 15;110(6):1235-40.
15. Spiess PE, Brown GA, Liu P, Tannir NM, Tu SM, Evans JG, Czerniak B, Kamat AM, Pisters LL. Predictors of outcome in patients undergoing postchemotherapy retroperitoneal lymph node dissection for testicular cancer. Cancer. 2006 Oct 1;107(7):1483-90.
16. Ravi P, Gray KP, O'Donnell EK, Tannir NM, Tu SM, Evans JG, Czerniak B, Kamat AM, Pisters LL. A meta-analysis of patient outcomes with subcentimeter disease after chemotherapy for metastatic non-seminomatous germ cell tumor. Ann Oncol. 2014 Feb;25(2):331-8
17. Heidenreich A, Thuer D, Polyakov S. Postchemotherapy retroperitoneal lymph node dissection in advanced germ cell tumours of the testis. Eur Urol. 2008 Feb;53(2):260-72
18. Logothetis CJ, Samuels ML, Trindade A, Johnson DE. The growing teratoma syndrome. Cancer. 1982 Oct 15;50(8):1629-35.
19. Motzer RJ, Amsterdam A, Prieto V, Sheinfeld J, Murty VV, Mazumdar M, Bosl GJ, Chaganti RS, Reuter VE. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol. 1998 Jan;159(1):133-8.
20. Baniel J, Foster RS, Gonin R, Messemer JE, Donohue JP, Einhorn LH. Late relapse of testicular cancer. J Clin Oncol. 1995 May;13(5):1170-6.
21. Gels ME, Nijboer AP, Hoekstra HJ, Sleijfer DT, Molenaar WM, Plukker JT, Droste JH, Schraffordt Koops H. Complications of the post-chemotherapy resection of retroperitoneal residual tumour mass in patients with non-seminomatous testicular germ cell tumours. Br J Urol. 1997 Feb;79(2):263-8
22. Baniel J, Sella A. Complications of retroperitoneal lymph node dissection in testicular cancer: primary and post-chemotherapy. Semin Surg Oncol. 1999 Dec;17(4):263-7.
(P647)