A Direct Oral Anticoagulant as a Cost Effective Alternative to Warfarin for Treatment of Provoked Venous Thrombosis
W Courtney1, E Groarke1, J Conway1, E Conway1, D Bourke1 , J Saunders2, M Watts3 , D O'Keeffe1,
1Department of Haematology, University Hospital Limerick, Dooradoyle, Limerick,
2Department of Maths and Statistics, University of Limerick, Limerick,
3Department of Medicine, University Hospital Limerick, Dooradoyle, Limerick, Ireland
Abstract
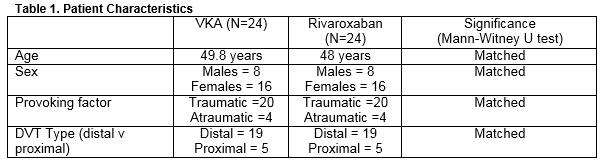
In Ireland, Warfarin is the primary anticoagulant prescribed in the secondary prevention of provoked DVT. We completed a comprehensive cost analysis of a trial group of 24 patients treated with Rivaroxaban (between November 2013 and December 2014), versus a control group treated with Warfarin (between January 2008 and November 2013). The groups were matched for gender (3/7 M/F ratio), DVT type (5 proximal, 19 distal DVTs), provoking factor (20 traumatic, 4 atraumatc), and age. We calculated the cost for each group based on drug administration and clinic costs (labour, sample analysis, and additional costs). Warfarin patients attended clinic 14.58 times; Rivaroxaban patients attended 2.92 times. Overall, the cost per patient on Rivaroxaban is €273.30 versus €260.68 with warfarin. This excludes patient costs which would further increase cost of Warfarin therapy.
Introduction
Deep venous thrombosis (DVT) is a common medical condition that contributes significantly to patient morbidity in both the inpatient and outpatient setting in Ireland and in the United Kingdom. Current guidelines recommend three months of anticoagulant therapy for all suitable patients with a provoked proximal DVT (involving the popliteal and/or more proximal veins)1. Traditionally, the agent of choice has been oral vitamin K antagonists (VKA) such as Warfarin. More recently, newer oral anticoagulant agents such as direct thrombin inhibitors and factor Xa inhibitors (Dabigatran etexilate (Pradaxa), Apixaban (Eliquis), and Rivaroxaban (Xarelto)) have been licensed in Ireland. Numerous studies have found these direct oral anticoagulants (DOACs) to be cost effective against the vitamin K antagonist Warfarin (VKA) for the prevention of stroke in atrial fibrillation (AF). The aim of this study is to compare the cost of prescribing and monitoring DOACs vs VKA in Ireland, in the treatment of provoked lower limb DVT, so that the results of this study can influence national anticoagulation guidelines in future.
Warfarin is a well-established anticoagulant and its production has a low cost base. DOACs currently retail at a much higher price2. Though it is significantly cheaper to produce and purchase, Warfarin has significant associated costs. When initiating Warfarin therapy, Bridging is required with expensive low molecular weight Heparin (LMWH) to ensure that further thrombotic events do not occur. This bridging is required until the International Normalised Ratio is therapeutic (in the range of 2.0 to 3.0). The patient must then have their INR monitored intensively throughout treatment. This monitoring requires the patient to regularly attend anticoagulation clinics for blood tests and dosage changes. Despite such intensive monitoring, maintaining a consistently therapeutic INR can be challenging. In contrast, Rivaroxaban, the DOAC which we selected to use in this study, does not require such intensive therapeutic monitoring, is prescribed in a fixed dosing regimen, and does not require a bridging period with LMWH at the outset of treatment.
In this study, we compared two cohorts of patients with provoked lower limb DVT requiring anticoagulation for a three month period. Our aim was to compare the outcomes and costs of anticoagulation between Warfarin and the factor Xa inhibitor Rivaroxaban. To do this, we collected our primary data from Limerick University Hospital which has a specialist thrombosis clinic running five days a week.
Methods
Consecutive patients who presented to the thrombosis clinic for anticoagulation for DVT therapy between December 1st 2013 and November 30th 2014 were included in the trial. We prospectively collected data on 26 patients who developed a provoked DVT and were treated with Rivaroxaban for a period of three months as per standard dosing 15mg BD for the first three weeks and 20mg OD for the subsequent 9 weeks (as per ACCP guidelines)1. Information was collected on a per visit basis throughout the duration of their treatment by a specialist anticoagulation nurse. Patients with an eGFR< 30 were deemed unsuitable to be commenced on Rivaroxaban, excluded from the trial, and commenced on Warfarin.
To obtain a comparable control group, we retrospectively analysed data stored on an electronic internal medicine ‘FileMaker Pro 12’ database. We identified patients who had been treated with Warfarin for three months for provoked DVT between January 1st 2008 and November 30th 2013. The patients from the control group were matched for gender, DVT type, and provoking factor. We reviewed the number of clinic visits and blood tests needed per patient over the three month treatment period. We analysed blood test records of each of the VKA patients using an electronic reporting system ‘ILAB’ to assess the proportion of time that each patient spent within the clinically therapeutic range of INR (2-3) during their 3 month treatment period.
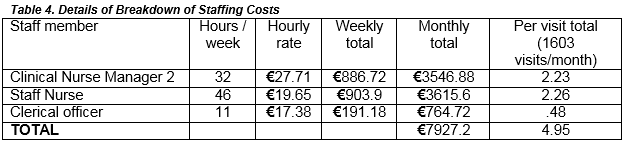
We identified the resources involved in the provision of VKA and Rivaroxaban respectively. VKAs, unlike Rivaroxaban, require the concomitant prescribing of low molecular weight heparin for a minimum of 5 days, or until a therapeutic INR is achieved. We calculated the cost of running the anticoagulant clinics and divided these costs into labour, drug costs, and additional costs which included the cost of materials needed to run clinic and sample analysis costs from the laboratory. In order to analyse the cost of labour, we logged the number of hours spent by the various members of staff who ran the anticoagulant clinics on a weekly basis. This included a Clinical Nurse Manager level 2, three staff nurses, and a clerical worker. We reviewed the Health Service Executive (HSE) website for information on the various staff member pay scales. To account for differences in pay for each staff member based on experience ( staff members annual remuneration increases incrementally annually based on number of years worked) we calculated the salary of each staff member based on the mid- point of the consolidated HSE salary scale for each rank of staff member.To attain the cost of supplying the drugs, we identified the cost of each drug from the HSE drug repayment scheme. To analyse material costs we calculated the cost of the needles used for phlebotomy in the clinics and the costs associated with informing patients of their follow up appointments. We calculated the cost to the hospital of analysing each blood sample based on current laboratory procedures.
Results:
Twenty-six patients with provoked DVTs were prospectively enrolled in the trial with provoked DVTs. Twenty-four patients completed 3 months of Rivaroxaban as per protocol and were eligible for inclusion. Two patients were excluded from the study as they did not complete their course of anticoagulation. In the first case, the patient’s medication was stopped as they were admitted to hospital for treatment of sepsis. While in hospital they were switched to heparin as an alternative anti-coagulant. In the second case, the patient was not eligible for inclusion because they developed non-specific upper and lower limb pains which they attributed to Rivaroxaban and switched over to warfarin, exiting the trial. Twenty-four matched controls were selected retrospectively from historical attendances as previously described.
Patients from the control group were matched for gender (16 females, 8 males per group with a 3/7 M/F ratio), and DVT type (5 proximal DVTs, 19 distal DVTs). Patients were also matched for “provoking factor” where the provoking factor was deemed to be the cause of the DVT and was either traumatic or atraumatic (20 atraumatic provoking factors, 4 traumatic provoking factors). We categorised provoking factors as traumatic if they occurred either directly as a result of trauma (fracture/ dislocation/ soft tissue injury) or due to plaster cast immobilisation following fracture. We categorised provoking factor as atraumatic if they were due to any other cause or combination of causes (medications, cigarette smoking, thrombophlebitis, high BMI, immobilisation, OCP). Both the trial and control groups had comparable age profiles. The mean age of the VKA group was 49.8 years and the mean age of the Rivaroxaban group was 48 years. These are well matched as per the Mann- Whitney U significance test.
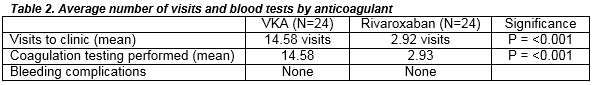
We analysed the number of clinic visits required by each patient over a 3 month period. VKA patients required significantly more clinic visits 14.58 (range 9-20; requiring a blood test on each visit) versus 2.92 in the trial group (range 2-4; requiring a blood test on each visit).
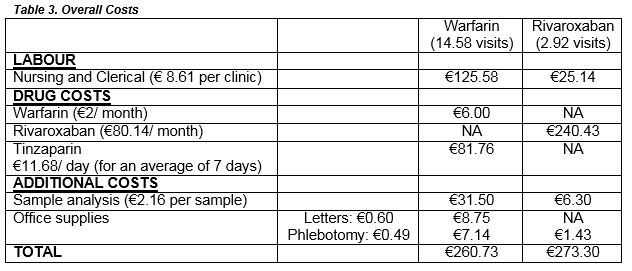
This study found that the overall cost to the health service per patient for 3 months of treatment was €260.68 for VKA therapy compared to €273.3 for Rivaroxaban therapy. The cost of labour per patient, per each anticoagulation clinic attended, was €8.61. Patients on Warfarin attended, on average, 14.58 times, which costed a total of €125.58 per patient. Patients on Rivaroxaban attended, on average, 2.92 times, which costed a total of €25.14 per patient. In terms of drug costs, each patient on warfarin cost a total of €87.76 ( cost of 3 month supply of warfarin and 11.68 day supply of Tinsaparin); each patient on Rivaroxaban cost €240.43 (3 month supply).
The Warfarin (and overall figures), do not include the cost to the patient of these multiple clinic visits such as transport, parking, and days off work.
For the 24 control patients, 285 INR results were recorded in our hospital over the three months of their therapy. Upon analysis of these, we found that 65.3 % (186/ 285) were within the clinically therapeutic range of 2.0- 3.0, 16.5 % (47/285) were supratherapeutic, and 18.2% (52/285) were subtherapeutic. However, there were no recurrences of thrombosis nor significant bleeding complications in either the VKA patients or the Rivaroxaban patients
Discussion
Patients being treated for provoked DVT with Rivaroxaban had similar overall costs to Warfarin for the Irish Health care system (€260.73 in the VKA group versus €273.30 in the Rivaroxaban group). Although the cost of Warfarin itself is low, additional costs, including the co-prescribing of low molecular weight heparin and intensive INR monitoring via outpatient phlebotomy clinics (mean 14.58 in the VKA group versus 2.93 in the Rivaroxaban group), are considerable. The funding and prescribing of the newer direct oral anticoagulants remains a topic of interest worldwide. Recent studies undertaken in a number of different countries have set out to compare DOACs with traditional VKAs and these have, for the most part, shown the cost effectiveness of DOACs over VKAs. The majority of these studies have looked at the use of anticoagulants for treatment of chronic atrial fibrillation4, 5, 6 with fewer investigating in DVT.7
This study indicates that in the setting of provoked DVT, with a predefined period of anticoagulation, Rivaroxaban is cost effective when compared to Warfarin in the Irish Healthcare system. This is primarily due to the fact that patients on warfarin require multiple visits to stabilise the INR particularly at the beginning of Warfarin treatment. This additional cost and burden on the patient must be taken into account by health systems when considering the most appropriate anticoagulant to use in this setting. Furthermore, a significant number of INR readings were outside of the therapeutic window despite being monitored in a specialist anticoagulation clinic. This is consistent with previous studies into INR control of patients on VKAs for atrial fibrillation8. The number of patients in our trial is too small to indicate whether this variable anticoagulation with warfarin affects outcome but previous studies have shown that the DOACs are safer with regard to major bleeding risk.
We did not analyse the cost of anticoagulation to the patients in our study. Previous studies which have quantified the cost of VKA therapy to the patient have calculated that the cost to a patient, when transport, parking and time off work are considered, is €48.509. Over a three month period, VKA patients in our trial will pay this amount 14.58 times causing them a significant financial cost when compared to Rivaroxaban patients who will only attend 2.92 times. We conclude that Rivaroxaban is cost effective compared to warfarin in patients requiring three months of anticoagulation for a provoked DVT in the Irish healthcare system.
Conflict of Interest
There is no conflict of interest with any of the authors associated with this paper.
Correspondence:
William Courtney
Department of Haematology, University Hospital Limerick, Dooradoyle, Limerick,
References:
1. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines CHEST 2012
2. HSE Drugs Repayment Scheme. Health Service Executive.
3. EINSTEIN Investigators. Oral Rivaroxaban for Symptomatic Venous Thromboembolism. N Engl J Med 2010; 363:2499-2510
4. Laliberté F, Pilon D, Raut MK, Nelson WW, Olson WH, Germain G, Schein JR, Lefebvre P. Is rivaroxaban associated with lower inpatient costs compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin. 2014;30:1–10
5. Kleintjens J, Li X, Simoens S, Thijs V, Goethals M, Rietzschel E, Asukai Y, Saka O, Evers T, Faes P, Vansieleghem S, De Ruyck M. Cost-effectiveness of rivaroxaban versus warfarin for stroke prevention in atrial fibrillation in the Belgian healthcare setting. 2013;31(10):909–918
6. Wang Y, Xie F, Kong MC, Lee LH, NgHJ, Ko Y. Cost-effectiveness of dabigatran and rivaroxaban compared with warfarin for stroke prevention in patients with atrial fibrillation. Cardiovasc Drugs Ther. 2014;28(6):575–585
7. Bamber L, Muston D, McLeod E, Guillermin A, Lowin J, Patel R . Cost-effectiveness analysis of treatment of venous thromboembolism with rivaroxaban compared with combined low molecular weight heparin/vitamin K antagonist. Thromb J. 2015 Jun 11;13:20. doi: 10.1186/s12959-015-0051-3. eCollection 2015.
8. Baker WL, Cios DA, Sander SD, Coleman CL. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009 Apr;15(3):244-52.
9. Walsh C, Murphy A, Kirby, A, Vaughan C. Retrospective Costing of Warfarin. Ir Med J. 2014 May;107 (5): 133-5
P466