Early Onset Neonatal E.Coli Sepsis
M. O’Rahelly1, A. Smith1, R. Drew2,3,4, N. McCallion1
1. Department of Neonatology, Rotunda Hospital
2. Clinical Innovation Unit, Rotunda Hospital
3. Irish Meningitis and Sepsis Reference Laboratory, Children’s University Hospital Temple St
4. Department of Microbiology, RCSI
Abstract
This was a retrospective case-control study over 14 years comparing characteristics of neonates who developed E.coli bacteraemia with matched infant controls whose mothers were colonised with E.coli on high vaginal swab but who did not develop bacteraemia. Data was obtained from maternal and neonatal charts, the laboratory data-base was analysed to identify possible risk factors for E.coli bacteraemia. 21 cases and 38 controls were identified. The data showed no difference in gender, maternal age, white cell count, or cord pH. Significant differences were found in gestation (33 vs. 39.5wks p<0.01), weight (1.64 vs. 3.08 kg p<0.001) and duration of preterm, pre-labour rupture of membranes (pPROM) (6 vs. 2.9 days p=0.04) between cases and controls. Risk factors for E.coli bacte-raemia were identified, most notably duration of pPROM. A re-evaluation of antimicrobial therapy in neonates with a maternal pPROM >5 days is advised.
Introduction
Neonatal sepsis is a major cause of neonatal morbidity and mortality in term and preterm infants. The timely identification of infants at risk of infection is of particular importance in the vulnerable preterm group1 and is a major focus of microbiological research in the Rotunda Hospital. E.coli accounts for approximately 14.1% of early onset sepsis, i.e. sepsis before 72 hours of age in our centre. E.coli is the second most common pathogen, along with coagulase negative Staphylococcus, after group B Streptococcus (GBS)2.
Internationally, E.coli causes approximately one quarter of early onset sepsis in newborns and carries a significant mortality, leaving one third of survivors with subsequent neurodevelopmental morbidity3,4. The morbidity associated with E.coli sepsis is more severe than that seen with group B Streptococcus septicaemia5. Prospective studies show that the majority of women who are colonised with E.coli on high vaginal swabs do not transmit the organism to their newborn infant6. In early 2016 E.coli was identified by our research group as a major research focus, aiming to identify infants at particular risk of developing bacteraemia. The early identification of infants at higher risk for bacteraemia would allow earlier antimicrobial intervention that could improve neonatal morbidity and mortality. Our group is concerned that by empirically targeting prophylactic cover for GBS we may inadvertently promote a reduction in commensal flora inhibition of E.coli, thereby increasing the risk of subsequent E.coli infection. The aim of this study was to evaluate the characteristics of neonates who develop E.coli bacteraemia and their mothers and compare such cases with controls. Controls were infants whose mothers carried E.coli on high vaginal swab (HVS) at the time of delivery but whose infants did not progress to develop E.coli bacteraemia.
Methodology
This was a retrospective case control study carried out in the Rotunda hospital Dublin, a tertiary neonatal referral centre with over 9,000 deliveries per annum. Our cases consisted of all early-onset E.coli sepsis over a 14 year period (2001-2014) which were confirmed on blood culture. Cases were identified using the hospital’s laboratory database which keeps record of all positive blood cultures. No exclusion criteria were applied to this group: any infant who had a positive blood culture for E.coli during the study period outlined was included in our cases. For our control group we collated a list of patients presenting during the same time period in chronological order of presentation. These comprised infants of mothers who had a positive HVS for E.coli within 24 hours of delivery of their child but did not develop subsequent E.coli bacteraemia. Patient hospital records were compared with birth records to ensure the positive swab was taken within 24 hours of delivery. We targeted a 2:1 control to case ratio to power our study and increase statistical significance. 38 controls fitted the inclusion criteria of a positive HVS for E.coli within 24 hours of delivery with an asymptomatic child.
Maternal and neonatal charts were examined to initially collect the data on both cases and controls. The electronic laboratory system was used to obtain microbiological and pathological data missing in patient records. Data on 61 variables was collected on both groups using excel and then analysed using SPSS v.24. The data on the case and control groups was then compared to identify differences between the two groups. We identified possible risk factors for neonatal E.coli sepsis from our collected data. Ethical approval was sought from the Rotunda hospital ethics committee and granted prior to study commencement.
Results
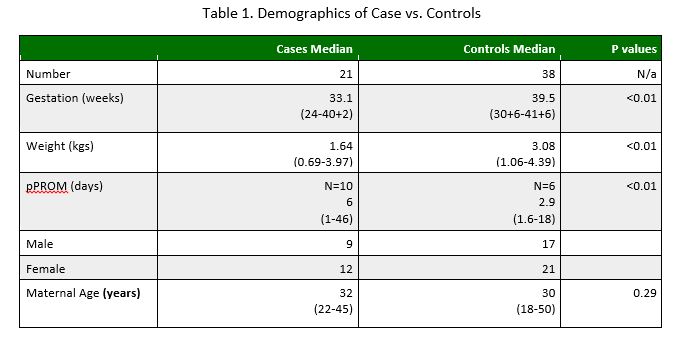
Between 2001 and 2014 21 neonates were confirmed with E.coli bacteraemia in the Rotunda Hospital, and a further 38 controls were identified with maternal E.coli colonisation on HVS who did not develop newborn infection. Significant differences were found between the two groups, which are summarised in table 1. The median (range) gestational age of the case group was 33.1 weeks (24-40+2) which was statistically different to the control group with a gestational age of 39.5 weeks (30+6-41+6), (p<0.01). The median (range) weight of our cases was 1.64kg (0.69-3.97) versus 3.08kg (1.06-4.39) in controls (p<0.01). pPROM (Pre-term Pre-labour rupture of membranes) was found in 47.6% of cases (n=10) versus 15.8% of controls (n=6). A significant finding was in relation to duration of pPROM. In our cases, pPROM was for a median of 6 days (1-46) whereas in the control group the median duration was 2.9 days (1.6-18) (P<0.01). There was no significant difference between groups in terms of gender, maternal age, and maternal white cell count (WCC) or cord pH.
Discussion
This study has identified a number of risk factors for the development of early-onset neonatal E.coli sepsis. These included an earlier gestation, lower birth weight and an increased duration of pPROM. The lower gestational age can explain the lower birth weights found in affected cases; however duration of pPROM is a separate and important risk factor.
We believe that patients who are premature, with a combination of low birth weight and pPROM can be considered at an increased risk of developing E.coli bacteraemia. These risk factors for E.coli bacteraemia are consistent with published literature looking at similar patient numbers, where intrapartum fever, pPROM, birth weight and onset of sepsis in the first day of life were associated with E.coli sepsis rather than GBS sepsis7.
The most important of the factors identified is duration of pPROM. The duration of pPROM could indicate a window for possible prophylactic pharmacological intervention. It may be necessary to reevaluate the choice of antimicrobial therapy in order to target gram negative bacteria in mothers experiencing pPROM with infants of a low gestational age. The risk associated with pPROM is significant as pathogens in neonatal sepsis are frequently acquired during passage through the birth canal8.This is especially important considering emergence of resistant strains of E.coli, some of which are now known to be both Ampicillin and Gentamicin resistant. The implications of resistant E.coli are significant as infections with E.coli have a worse outcome in the neonatal population. To date there do not seem to be identifiable risk factors for cohorting women into groups which may be colonised with resistant strains of E.coli9,10. In the context of pPROM, antenatal antibiotics are administered in our centre if the duration of rupture of membranes is >18hours. Previous studies have found that while this is effective in eradication of group B Streptococcus it is rarely effective at preventing E.coli infections11. A concern with using Erythromycin for pPROM is that it can lead to eradication of non-pathogenic commensal flora in the vagina, leading to over growth of Gram negative organisms such as E.coli. The depletion of Lactobacilli in the vagina has been shown to be exacerbated in pPROM through use of Erythromycin12. Thus to prevent early onset Gram negative sepsis in the setting of pPROM, it may be necessary to consider Lactobacilli supplementation for these women. This is increasingly important given the rise of antimicrobial resistance in European E.coli strains13. A recent study looking at over two decades of data in Spain found that there is growing evidence of increased resistance to Gentamicin and Ampicillin amongst E.coli strains, further stressing the vital importance of stringent antimicrobial stewardship. They also found that in their cohort of patients the incidence of E.coli sepsis was increasing. This emphasises that empiric antibiotic therapy may have to be reviewed where risk factors exist for E.coli neonatal infection14.
The limitations of our study include that it was a retrospective study and as such we had incomplete data sets for certain data points in both our case and control populations. The number of both cases and controls in our study was also a limiting factor, we had originally targeted a 2:1 control to case ratio but when we reviewed our database we did not have sufficient numbers to achieve this. However as one of largest maternity centres in Ireland we feel the case number cannot be overlooked. During the study period approximately 112,000 live births occurred in our centre and we feel on this basis our study provides information on a realistic rate of early-onset E.coli bacteraemia. Our controls and cases were not matched for gestation or age as the two groups were too heterogeneous; however the two groups were matched as all mothers were E.coli positive on HVS at the time of delivery. As a lot of our data was collected from a chart review we relied on adequate documentation, it is possible that some outcomes were either not documented or documented incorrectly. Due to our sample size it is also difficult to draw any firm conclusions from our data; we however hope that this will contribute to the already existing pool of data which may be used in the future for review.
For infants born prematurely, especially in the setting of pPROM with latency to delivery of a few days, additional cephalosporin treatment should be considered in order to treat possible Gram-negative sepsis and meningitis.
Conflict of Interest
The authors declare that there is no conflict of interest.
Corresponding Author
Mark O’Rahelly,
Department of Neonatology,
Rotunda Hospital,
Dublin
Email: [email protected]
References
1. Drew RJ, Fonseca-Kelly Z, Eogan M. A Retrospective Audit of Clinically Significant Maternal Bacteraemia in a Specialist Maternity Hospital from 2001 to 2014. Infect Dis Obstet Gynecol 2015;2015:518562.
2. Huggard D, Drew R, McCallion N. Neonatal Bacteraemia Among 112,360 Live Births. Ir Med J 2016;109(9):467.
3. Jones B, Peake K, Morris AJ, McCowan LM, Battin MR. Escherichia coli: a growing problem in early onset neonatal sepsis. Aust N Z J Obstet Gynaecol 2004;44(6):558-61.
4. Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev 2014;27(1):21-47.
5. Mayor-Lynn K, Gonzalez-Quintero VH, O'Sullivan MJ, Hartstein AI, Roger S, Tamayo M. Comparison of early-onset neonatal sepsis caused by Escherichia coli and group B Streptococcus. Am J Obstet Gynecol 2005;192(5):1437-9.
6. Chan GJ, Lee AC, Baqui AH, Tan J, Black RE. Risk of early-onset neonatal infection with maternal infection or colonization: a global systematic review and meta-analysis. PLoS Med 2013;10(8):e1001502.
7. Tsai CH, Chen YY, Wang KG, Chen CY, Chen CP. Characteristics of early-onset neonatal sepsis caused by Escherichia coli. Taiwan J Obstet Gynecol 2012;51(1):26-30.
8. Jiang JH, Chiu NC, Huang FY, Kao HA, Hsu CH, Hung HY, Chang JH, Peng CC. Neonatal sepsis in the neona-tal intensive care unit: characteristics of early versus late onset. J Microbiol Immunol Infect 2004;37(5):301-6.
9. Joseph TA, Pyati SP, Jacobs N. Neonatal early-onset Escherichia coli disease. The effect of intrapartum ampicillin. Arch Pediatr Adolesc Med 1998;152(1):35-40.
10. Friedman S, Shah V, Ohlsson A, Matlow AG. Neonatal escherichia coli infections: concerns regarding re-sistance to current therapy. Acta Paediatr 2000;89(6):686-9.
11. Hershkovich-Shporen C, Bardenstein R, Blickstein I, Shinwell ES, Flidel-Rimon O. Maternal intrapartum antibiotic treatment continues to exert a bactericidal effect on the umbilical cord and peripheral venous blood of newborn infants. Acta Paediatr 2017;106(11):1767-71.
12. Stokholm J, Schjorring S, Eskildsen CE, Pedersen L, Bischoff AL, Folsgaard N, Carson CG, Chawes BL, Bon-nelykke K, Molgaard A, Jacobsson B, Krogfelt KA, Bisgaard H. Antibiotic use during pregnancy alters the commen-sal vaginal microbiota. Clin Microbiol Infect 2014;20(7):629-35.
13. Allocati N, Masulli M, Alexeyev M, Di Ilio C. Escherichia coli in Europe: An Overview. International Jour-nal of Environmental Research and Public Health 2013;10(12):6235-54.
14. Mendoza-Palomar N, Balasch-Carulla M, Gonzalez-Di Lauro S, Cespedes MC, Andreu A, Frick MA, Linde MA, Soler-Palacin P. Escherichia coli early-onset sepsis: trends over two decades. Eur J Pediatr 2017;176(9):1227-34.
P868
