The Predictive Ability of Pre-Operative Magnetic Resonance Imaging to Detect Pathological Outcomes in Prostate Cancer
Nason GJ1, Selvarajah L2, O’Connor EM1, O’Kelly J1, Considine SW1, Moss B1, MacMahon D3, Heneghan J4, Meyer N5, Buckley J2, O’Regan K2, O’Brien MF1,3.
1.Department of Urology, Cork University Hospital, Wilton, Cork, Ireland
2.Department of Radiology, Cork University Hospital, Wilton, Cork, Ireland
3.Department of Urology, University Hospital Waterford, Waterford, Ireland
4.Department of Radiology, University Hospital Waterford, Waterford, Ireland
5.Department of Histopathology, Cork University Hospital, Wilton, Cork, Ireland
Abstract
Aims
Accurate preoperative knowledge of tumour stage is important in preoperative planning at radical prostatectomy (RP). The aim of this study was to assess the predictive ability of multiparametric MRI for detecting pathological outcomes.
Methods
A retrospective review was performed of all patients who underwent RP over a 4 year period.
Results
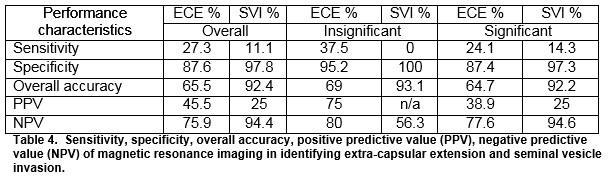
Preoperative MRI was reported as showing T3 or T4 disease in 26(17.9%) out of 145 patients undergoing RP. Of these, 10(6.9%) had ECE (extra-capsular extension) and 1(0.7%) had SVI (seminal vesicle invasion) on final histology. The sensitivity and specificity of MRI for detecting ECE were 27.3% and 87.6%, respectively. The sensitivity and specificity of MRI for detecting SVI were 11.1% and 97.8%, respectively. The positive predictive values for determining ECE and SVI were 45.5% and 25%, respectively and negative predictive values were 75.9% and 94.4%.
Conclusion
MRI has good specificity but poor and heterogeneous sensitivity for predicting T3 disease in RP specimen.
Introduction
Prostate cancer (CaP) is the most common solid organ neoplasm in males1.There is lack of consensus regarding the optimal diagnostic, staging and treatment options for CaP. Several factors such as age, comorbidities, PSA, Gleason grade, tumour volume and local extension of the disease influence management2. Pathological stage T3 disease (American Joint Committee on Cancer (AJCC) Prostate cancer staging manual 7th edition) is classified as extra-capsular extension (ECE) and/or seminal vesicle invasion (SVI). Prediction of T3 disease by digital rectal examination and transrectal ultrasound (TRUS) is known to have low accuracy3,4. Accurate preoperative knowledge of tumour stage and ECE are important in preoperative planning to achieve the best surgical, oncological and functional results. ECE is important with regards to the decision making process when considering a nerve sparing approach and the extent of surgical margins5,6. Multiparametric magnetic resonance imaging (mpMRI) use is expanding as a diagnostic technique in CaP staging and ECE risk assessment7. Pathologists prepare the prostatectomy specimen as axial tumour maps which allow direct correlation with the pre-operative MRI 8.
Sensitivity and specificity of mpMRI for detecting ECE are widely variable and are influenced by magnetic field strength (1.5 Tesla vs 3 Tesla), the use of an endorectal coil and MRI parameters used9. Furthermore, inter-observer variation in MRI reporting can occur. To reduce this variation a more structured uniform reporting and scoring system (PIRADS) has been devised and implemented in many centres10.The aim of this study was to assess the predictive ability of pre-operative MRI for detecting pathological outcomes at radical prostatectomy (RP) in our unit.
Methods
A retrospective review was performed of all patients who underwent RP by a single fellowship trained Consultant Urologist in a tertiary referral hospital between April 2012 and January 2016. Demographic, clinical and pathological details on these patients were collected. A standard radical retropubic prostatectomy was performed via a 10cm lower midline incision. All patients underwent a staging MRI preoperatively- some of these were performed outside of the central institution. All cases were however reviewed centrally (CUH) pre- and post-operatively at the institutional MDM and imaging was reviewed by either of two fellowship trained radiologist with experience in standardised prostate MRI reporting (PIRADS 2). All scans were performed on either 1.5T (GE Optima 1.5T 450w and Siemens 1.5T Magnetom, Siemens healthcare, Munich, Germany) or 3T MRI scanners (GE Discovery 750w 3.0T 70 cm bore, GE healthcare, Fairfield, Connecticut, US). The 3T MRI unit was installed during the study period and therefore scans performed early in the series were 1.5T while subsequent scans were performed at 1.5T or 3T.
At our unit, we routinely perform axial and coronal T2-weighted sequences, axial diffusion-weighted sequence (b-value 1000s/mm2), and axial T1-weighted sequence through the pelvis. We do not use an endorectal coil and a standard body coil is used at both 1.5T and 3T. We do not routinely perform dynamic contrast enhanced sequences or MR spectroscopy. Histopathological characteristics from the RP specimen were compared to radiological staging from the finalised imaging report and the outcome of the imaging review discussion at the multidisciplinary meeting (MDM). All histological specimens were independently reviewed by at least two Consultant Histopathologists. Histology specimens were reported according to the American Joint Committee on Cancer (AJCC) Prostate cancer staging manual 7th edition. Histopathologically, ECE is defined as a cancer that extends through the prostatic capsule into the periprostatic adipose tissue. ECE is further subdivided into focal ECE and established ECE. Focal ECE refers to the presence of no more than a few malignant cells immediately outside the capsule (i.e. within one or two step-sections). Established ECE refers to all other cases of ECE and includes more extensive presence of malignant cells outside the capsule11.
CaP was classified as insignificant or significant disease. Insignificant disease was described as low risk by the NCCN risk group stratification (PSA <10 ng/mL, Gleason score ≤6, clinical stage T1c or T2a) where as significant disease was described as intermediate (PSA >10 but < 20 ng/mL, Gleason score=7, clinical stage T2b) or high risk (PSA>20 ng/mL, Gleason score ≥8, clinical stage T2c/T3). Diagnostic accuracy of MRI scan (designed to distinguish ECE and SVI)) can be quantified by the measures such as sensitivity and specificity, overall accuracy, positive and negative predictive values (PPV, NPV). Following the Royal College of Radiologists in the UK, microscopic or focal ECE on radical prostatectomy specimen is considered pathologically non-ECE with regards to MRI audit. Thus, histological focal ECE that was undetected by MRI need not be considered a discrepancy 12. Statistical analysis was performed using Pearson’s x2 test and the Fisher exact test to compare categorical variables (SPSS Version 21.0 New York, USA). A p-value of 0.05 was considered statistically significant.
Results
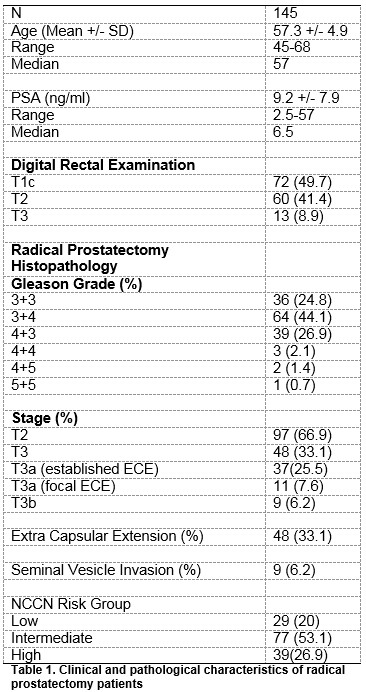
During the study period, 145 RP were performed. 97 (66.9%) patients had organ confined (pathological T2 disease) while 48 (33.1%) had evidence of ECE (pathological T3 disease). Of the 48 patients with pT3 disease, 37 (77.1%) had established ECE and 11(22.9%) had focal ECE. Clinical and pathological characteristics are detailed in Table 1. 129 (89%) of the MRI staging scans were performed at 1.5T while 16 (11%) scans were at 3T. Pre-operative MRI detected ECE (T3 disease) in 22 patients and seminal vesicle invasion (SVI) in 4 patients. Of these, 10 had ECE and 1 had SVI on final histology. MRI upstaged 12 patients regarding ECE and 3 patients regarding SVI, Tables 2 and 3.
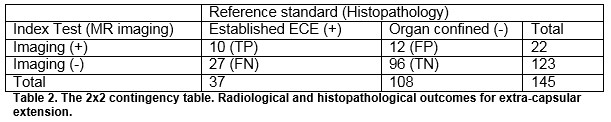
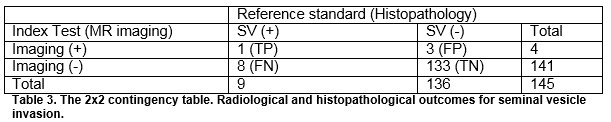
The overall sensitivity and specificity of MRI for detecting ECE were 27.3% and 87.6% respectively. The overall sensitivity and specificity of MRI for detecting SVI were 11.1 and 97.8%, respectively. The positive predictive values for determining ECE and SVI were 45.5% and 25%, respectively and negative predictive values were 75.9% and 94.4%. For insignificant disease, the NPV for ECE and SVI was 80 and 56.3%, respectively. For significant disease, the NPV for ECE and SVI was 77.6% and 94.6%, respectively, Table 4.
Discussion
Our study demonstrates a relatively low accuracy of MRI for predicting pathological outcomes at RP. At present in our institution, all patients with CaP undergo a staging MRI in advance of surgery. The presence of T3 disease is one of the key factors in the joint decision making process with the patient- therefore the accuracy of MRI is paramount.
There remains lack of consensus regarding the optimal sequences for prostate MRI. In a meta-analysis, da Silva et al., demonstrated low sensitivity (49%) and specificity (82%) for MRI in detecting ECE and SVI, similar to our findings13. In contrast to our study, their analysis focused solely upon 1.5 Tesla MRI with an endorectal coil. Chabanova et al. compared conventional MRI and spectroscopy and perfusion methods in the diagnosis of prostate cancer. They demonstrated that the combination of conventional MRI, spectroscopy and perfusion could diagnose all patients with CaP, however it was associated with a higher cost and was more time consuming to perform and report14. Yoshizako et al. assessed the combination of conventional MRI with diffusion and perfusion. The combination of conventional MRI and diffusion showed sensitivity of 80% and specificity of 87%, and when combined with perfusion showed sensitivity of 69% and specificity of 68%. The combination of the three methods showed sensitivity of 69% and specificity of 93%15.
Oon et al., in the only other published Irish series, demonstrated more favourable sensitivity (75%) and specificity (100%) for detecting pathological outcomes in a smaller surgical series. Interestingly- the majority of that study only had conventional MRI- without diffusion weighted imaging (DWI) or dynamic contrast enhancement (DCE)16, similar to our series. Contrary to our study, these MRI scans were performed prior to prostate biopsy. In the setting of a rising level of PSA and negative transrectal prostate biopsies, NiMhurchu et al, demonstrated significant correlation between DWI and targeted peripheral zone prostate lesions for detecting prostate cancer17.
The correlation between histopathological lesions and MRI findings is difficult to determine, especially due to the variations between MR cross -sections and prostatectomy slices, and the shrinkage of the surgical specimen during histopathological processing18. The suspicion of ECE at MRI is made by direct and indirect signs, such as the presence of solid tissue in the periprostatic fat, irregular bulging of the prostatic capsule and obliteration of the retroprostatic angle, as well as non-specific signs such as capsular thickening, capsular retraction and regular bulging of the capsule19.
There is well documented inter-observer variability regarding the interpretation and reporting of MRI therefore standardised reporting protocols by dedicated uro-radiologists are useful. The uptake of MRI as a diagnositic tool has previously been slow due to lack of standardised reporting of results20. To standardize the evaluation and reporting of prostate MRI, the European Society of Urogenital Radiology (ESUR) published guidelines based on an expert consensus in 2012, termed the Prostate Imaging Reporting and Data System (PIRADS). This guideline provides explicit and standardised criteria for Likert-scoring of multiparametric sequences (T2w, diffusion-weighted imaging (DWI), dynamic contrast enhanced imaging (DCE) and MR-spectroscopy)10. The accuracy of mpMRI and PIRADS scoring has not only been established for biopsy specimen, but also for histopathologic correlation using prostatectomy specimen. Prior to the PIRADS era, Isebaert et al. found a sensitivity of 58.5% for CaP detection21, which may account for the poor sensitivity rates seen in our cohort. Recent publications demonstrate detection rates of significant CaP between 80-96% for MRI compared to whole-mount sections22,23.
Somford et al. found a NPV of 87.7% for ECE in patients with low-risk CaP and a PPV for ECE in high-risk patients of 88.8%6. Marcus et al. investigated the impact of preoperative MRI on NCCN risk group classification in a cohort of 71 patients and found that 16.9% of patients were upstaged by MRI, mostly from intermediate- to high-risk24. McClure et al. found a change in the initial surgical plan in 27% of patients, analyzing a cohort of 104 consecutive men. In their study of a series of laparoscopic RP, the surgical plan was changed to a nerve-sparing technique in 61% and to a non-nerve-sparing in 39%5.
The Royal College of Radiologists UK offers some suggestions to improve the accuracy for preoperative MRI- the use of an endorectal coil, increased interval between TRUS biopsy and MRI, repeating MRI if significant artefact or post biopsy haemorrhage and limiting prostate MRI reporting to dedicated uro-radiologists10.
Our study is limited its retrospective analysis of a small surgical series performed by a single surgeon. These results may not be representative of the whole population as many patients who had prostate MRI were managed with active surveillance or radiotherapy and these were not included as we would not be able to correlate with final histology. The pathological outcomes (ECE and SVI) were only present in a small number of patients which may impact the analysis. All MRI scans were not performed in the same institution and the use of the 3T machine was not available until late in the series. Given the unit is a tertiary referral centre some patients had scans performed peripherally at outside institutions with slight differences in acquisition protocols and sequences. All pre-operative imaging was however reviewed at the MDM in the cancer centre by one of two radiologists with training in prostate MRI interpretation. Over the time period of this study, the PIRADS standardised scoring system was not routinely applied when reporting prostate MRI.
The results of our retrospective cohort study show a relatively a relatively low accuracy of mpMRI for predicting T3 disease in the RP specimen. The predictive ability of mp-MRI was lower in patients with low risk disease, while accuracy improved in high risk patients. The use of higher magnetic field strength MRI at 3T, use of endorectal coil, and specialist interpretation/ reporting (PIRADS 2) may all contribute to increasing the accuracy of preoperative staging of prostate cancer, and future prospective studies should focus on these areas.
Conflicts Of Interest
There are no conflicts of interest to report.
Correspondence
Mr Gregory Nason, FRCS Urol., Specialist Registrar in Urology, Department of Urology, Cork University Hospital, Wilton, Cork, Ireland
Email: [email protected]
Phone: +353-21-48034567
References
1. Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni F; European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011 Jan;59(1):61-71.
2. Chodak GW. The role of watchful waiting in the management of localized prostate cancer. J Urol. 1994 Nov;152(5 Pt 2):1766-8.
3. Mullerad M, Hricak H, Kuroiwa K, Pucar D, Chen HN, Kattan MW, Scardino PT. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol. 2005 Dec;174(6):2158-63.
4. Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005 Apr;95(6):751-6.
5. McClure TD, Margolis DJ, Reiter RE, Sayre JW, Thomas MA, Nagarajan R , Gulati M, Raman SS. Use of MR imaging to determine preservation of the neurovascular bundles at robotic-assisted laparoscopic prostatectomy. Radiology. 2012 Mar;262(3):874-83.
6. Somford DM, Hamoen EH, Fütterer JJ, van Basten JP, Hulsbergen-van de Kaa CA, Vreuls W, van Oort IM, Vergunst H, Kiemeney LA, Barentsz JO, Witjes JA. The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low, intermediate and high risk prostate cancer. J Urol. 2013 Nov;190(5):1728-34.
7. Boesen L, Chabanova E, Løgager V, Balslev I, Mikines K, Thomsen HS. Prostate cancer staging with extracapsular extension risk scoring using multiparametric MRI: a correlation with histopathology. Eur Radiol. 2015 Jun;25(6):1776-85
8. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL; ISUP Grading Committee.The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005 Sep;29(9):1228-42.
9. Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, Scheenen TW, Vos PC, Huisman H, van Oort IM, Witjes JA, Heerschap A, Fütterer JJ. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011 Oct;261(1):46-66.
10. Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, Rouviere O, Logager V, Fütterer JJ; European Society of Urogenital Radiology. ESUR prostate MR guidelines 2012. Eur Radiol 2012 Apr;22(4):746-57.
11. Eggener SE, Scardino PT, Carroll PR, Zelefsky MJ, Sartor O, Hricak H, Wheeler TM, Fine SW, Trachtenberg J, Rubin MA, Ohori M, Kuroiwa K, Rossignol M, Abenhaim L; International Task Force on Prostate Cancer and the Focal Lesion Paradigm. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007 Dec;178(6):2260-7.
12. https://www.rcr.ac.uk/audit/how-accurate-mri-staging-prostate-cancer
13. Silva RC, Sasse AD, Matheus WE, Ferreira U. Magnetic Resonance Image in the diagnosis and evaluation of extra-prostatic extension and involvement of seminal vesicles of prostate cancer: a systematic review of literature and meta-analysis. Int Braz J Urol. 2013 Mar-Apr;39(2):155-66.
14. Chabanova E, Balslev I, Logager V, Hansen A, Jakobsen H, Kromann-Andersen B, Norgaard N, Horn T, Thomsen HS. Prostate cancer: 1.5T endocoil dynamic contrast-enhanced MRI and MR spectroscopy-- correlation with prostate biopsy and prostatectomy histopathological data. Eur J Radiol. 2011 Nov;80(2):292-6.
15. Yoshizako T, Wada A, Hayashi T, Uchida K, Sumura M, Uchida N, Kitagaki H, Igawa M. Usefulness of diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging in the diagnosis of prostate transition-zone cancer. Acta Radiol. 2008 Dec;49(10):1207-13.
16. Oon SF, Power SP, Kelly JS, McDermott V, Ryan P, Ryan PC. The accuracy of magnetic resonance imaging in prostate cancer staging: a single-institution experience. Ir J Med Sci. 2015 Jun;184(2):313-7.
17. NiMhurchu E, O'Kelly F, Murphy IG, Lavelle LP, Collins CD, Lennon G, Galvin D, Mulvin D, Quinlan D, McMahon CJ. Predictive value of PI-RADS classification in MRI-directed transrectal ultrasound guided prostate biopsy. Clin Radiol. 2016 Apr;71(4):375-80
18. Rud E, Klotz D, Rennesund K, Baco E, Berge V, Lien D, Svindland A, Lundeby E, Berg RE, Eri LM, Eggesbø HB. Detection of the index tumour and tumour volume in prostate cancer using T2-weighted and diffusion-weighted magnetic resonance imaging (MRI) alone. BJU Int. 2014 Dec;114(6b):E32-42.
19. Yu KK, Hricak H, Alagappan R, Chernoff DM, Bacchetti P, Zaloudek CJ. Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: multivariate feature analysis. Radiology. 1997 Mar;202(3):697-702.
20. Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, Heijmink SW, Hoskin P, Kirkham AP, Padhani AR, Persad R, Puech P, Punwani S, Sohaib A, Tombal B, Villers A, Emberton M. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imaging. 2013 Jan;37(1):48-58.
21. Isebaert S, Van den Bergh L, Haustermans K, Joniau S, Lerut E, De Wever L, De Keyzer F, Budiharto T, Slagmolen P, Van Poppel H, Oyen R. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J Magn Reson Imaging. 2013 Jun;37(6):1392-401.
22. Baco E, Ukimura O, Rud E, Vlatkovic L, Svindland A, Aron M, Palmer S, Matsugasumi T, Marien A, Bernhard JC, Rewcastle JC, Eggesbø HB, Gill IS. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2015 Apr;67(4):787-94
23. Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS, Huang J, Margolis DJ, Raman SS, Reiter RE. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015 Mar;67(3):569-76.
24. Marcus DM, Rossi PJ, Nour SG, Jani AB. The impact of multiparametric pelvic magnetic resonance imaging on risk stratification in patients with localized prostate cancer. Urology. 2014 Jul;84(1):132-7.
P790
