An Approach to Optimise Therapeutic Vancomycin Dosage in a Haemodialysis Population
H Gunning, G Taylor, A Smyth, G Mellotte, J Fennell, P Murphy, P Lavin, C Wall
Trinity Health Kidney Centre, AMNCH, Tallaght, Dublin 24
Abstract
Haemodialysis patients are at risk of gram-positive bacteraemia and commonly require intravenous vancomycin. Intravenously administered vancomycin is primarily excreted by the kidney and exhibits complex pharmacokinetics in haemodialysis patients; achieving therapeutic levels can be challenging. An audit in our unit showed current practises of vancomycin administration resulted in a high proportion of sub-therapeutic levels. A new protocol was developed with fixed weight-based loading and subsequent dosing guided by pre-dialysis levels, target levels were 10-20mg/L. Its effectiveness was prospectively evaluated between 24th September 2012, and 8th February 2013. During this period 25 patients commenced vancomycin, 15 were included. In total, 112 vancomycin levels were taken, 94 (84%) were therapeutic, this was a significant improvement compared to previous practise (odds ratio 5.4, CI 3.1-9.4, p < 0.0001). In conclusion, our study shows this protocol can consistently and reliably achieve therapeutic vancomycin levels.
Introduction
Haemodialysis patients using tunnelled dialysis catheters are at risk of bacteraemia1. It is the second leading cause of mortality in this group of patients, with an annual risk of 8-14%2,3. The most common causative organisms in catheter-related bloodstream infections are Gram-positive4, Staphylococcus aureus and coagulase-negative staphylococci being the prime culprits5. To account for the increasing proportion of staphylococcal species with reduced susceptibility to ß-lactam antibiotics2, first-line regimens include vancomycin whilst awaiting microbiological identification and sensitivities of the causative organism.
Vancomycin is a bactericidal glycopeptide antibiotic, when intravenously administered it is primarily renally cleared (90%); 10% undergoes hepatic clearance6. Data suggests that non-renal clearances of vancomycin are also reduced in renal impairment. It is hypothesised uraemic toxins inhibit vancomycin metabolism7. As creatinine clearance falls, vancomycin clearance reduces in a linear fashion8. With normal renal function the half-life of vancomycin is 5-8 hours, this increases to 100-200 hours in end-stage kidney disease9. Vancomycin exhibits a narrow therapeutic window and whilst overdosing poses the risks of toxicities, including nephrotoxicity10 and ototoxicty11, under-dosing risks treatment failure and emergence of resistant organisms12-14. It exerts its bactericidal action only when its concentration is above the minimal inhibitory concentration (MIC). In practical terms, this requires a trough level in the 10-20mg/L range. Levels over 10mg/L are required to reduce the risk of resistance developing during therapy13.
Although vancomycin is not significantly cleared using low flux dialysis membranes, higher clearance is achieved with high flux membranes or haemodiafiltration7,15. The pharmacokinetics of vancomycin in haemodialysis are complex and triphasic16. With contemporary high-flux membranes, phase 1 describes a rapid clearance phase during dialysis; in a typical 4-hour session, the serum extraction rate is approximately 30-46%12,17-20. Phase 2 shows a sharp peak in vancomycin levels with dosing followed by subsequent redistribution, and phase 3 is the inter-dialytic interval with slow clearance and a serum half-life of 100-200 hours21,22.
Multiple variables may affect vancomycin levels achieved including patient weight, dialysis duration, type of filter used, blood and dialysate flows, inter-dialytic interval and residual renal function16,23. Given these factors, achieving therapeutic vancomycin levels, whilst avoiding toxicity, is challenging. Although weight-based loading is known to be superior to fixed dosing12,18,19, there are no clear guidelines for maintenance dosing.
Trinity Health Kidney Centre is based in Tallaght Hospital, Dublin, Ireland, a tertiary referral hospital with a Haemodialysis unit. The unit uses High Flux FX-series dialysers24, both haemodialysis and haemodiafiltration techniques are employed. An audit evaluated the effectiveness of the practice of vancomycin administration in haemodialysis patients in 2009. Of concern, 64.0% of levels were below target range and of these 66.3% did not receive a vancomycin dose on this day. Due to local resources, a patient’s vancomycin level may not be available during the dialysis session in which it was taken, limiting further dosing during this session; it was hypothesised that this was central to the high number of days with sub-therapeutic levels.
These results prompted the development of a new protocol aiming to ensure consistent gram-positive antimicrobial cover, whilst minimising the risk of toxicity, in patients with end-stage renal disease undergoing haemodialysis. A protocol was designed which, after initial loading, allowed vancomycin to be administered by nursing staff guided by levels, without consulting the physician. Further to this, doses would be based on vancomycin levels from the previous dialysis session; thus ensuring their availability.
Methods
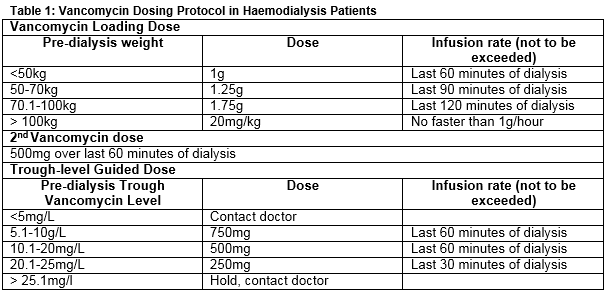
This was a prospective study of a new vancomycin administration protocol performed at our institution. The new protocol was reviewed and approved by the hospital’s Drugs and Therapeutics Committee. The literature was reviewed and communication was undertaken with peers on a national level, to ascertain their practices surrounding dosing and therapeutic drug monitoring. A new protocol for vancomycin administration in haemodialysis patients was developed with a clearly designed new process for dosing and monitoring. There was clear staff assignation of process roles, which involved a greater degree of nurse involvement in application of the agreed protocol. A weight-based, physician prescribed, loading regimen was designed, and a predefined second dose for all patients was set. These doses were to be prescribed on the once-only section of the prescription chart. Subsequent to this, each dose was guided by the pre-dialysis trough vancomycin level, taken at the previous haemodialysis session, aiming for levels of 10-20mg/L. Unless this trough level was outside target range, the dose to be delivered was protocolised and delivered by nursing staff without physician involvement. When levels were outside target, the physician was alerted to prescribe further dosing in an effort to attain the desired trough level (Table 1). This protocol was prescribed on the regular medication section of the prescription chart.
The study was performed between 24/9/2012 and 8/2/2013 and data was collected prospectively in all patients who received vancomycin, by an independent audit team. Patients were excluded if they commenced vancomycin at a different institution and if they had a significant degree of residual renal function, defined by requiring haemodialysis less than three times weekly. Attention was paid to trough vancomycin levels achieved with the weight-based loading protocol and levels achieved overall during the period of vancomycin treatment. Vancomycin Hydrochloride and the AxSYM Vancomycin assay25 were used during both study periods. Data collection was performed using a Microsoft Excel pro forma and statistical analysis using JMP 10 software (SAS, Cary, North Carolina, USA).
Results
During the study period 25 patients were commenced on vancomycin therapy. Seven patients were excluded – three discontinued therapy before the first trough level, two initiated vancomycin at an alternative unit, one had significant residual renal function and in one case the protocol was deliberately not followed. Of the 18 patients included, 17 received dialysis three times weekly and one four times weekly; 11 patients were treated with haemodialysis, five with haemodiafiltration and two with a combination. Seven patients did not receive weight-based loading as per protocol. Subsequent to loading the protocol was followed completely in all 18 cases.
Patients administered vancomycin with at least one trough level taken
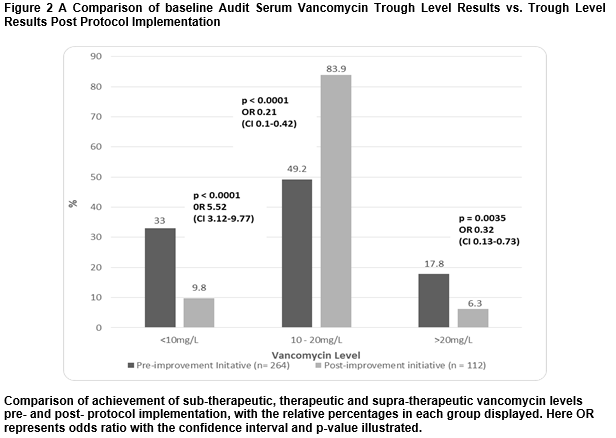
The median treatment duration was 15 (IQR 15.5) days. Two patients continued vancomycin beyond the study period and four continued to have only one trough level taken. The median number of doses received per patient was eight (IQR 5.8). The total number of vancomycin levels taken, including those taken outside of the protocol guidelines, was 112 and the median number per treatment course was 6.5 (IQR 4.8). The median vancomycin level achieved was 13.3mg/L (IQR 3.9). Median levels achieved at each treatment point in a course are shown in figure 1. Of the levels achieved 83.9% were therapeutic, 9.8% sub-therapeutic and 6.3% supra-therapeutic. Compared to previous practice, the new protocol had a significantly higher proportion of therapeutic levels (odds ratio 5.4, CI 3.1-9.4, p < 0.0001) and significantly lower proportions of sub-therapeutic (odds ratio 0.2, CI 0.1-0.4, p <0.0001) and supra-therapeutic (odds ratio 0.3, CI 0.1-0.7, p = 0.0035) levels, (figure 2).
Weight-based loading
Eleven patients (61.1%) received weight-based loading with a median vancomycin level after loading of 15.0mg/L (IQR 5.7). Of the levels achieved 72.7% were therapeutic, 9.1% sub-therapeutic and 18.2% supra-therapeutic. Of the seven patients (38.9%) who did not receive weight-based loading, the median vancomycin level was 15.2mg/L (IQR 8.6). Almost 43% of levels were therapeutic, 42.9% sub-therapeutic and 14.3% supra-therapeutic. The differences in the weight-based loading and non-weight based loading groups in achieving therapeutic, sub-therapeutic and supra-therapeutic levels were not statistically significant.
Discussion
Our study shows a significant improvement in practice of vancomycin administration in haemodialysis patients. The protocol reliably and consistently achieves therapeutic vancomycin levels, with reduced incidences of both sub-therapeutic and supra-therapeutic levels when compared to previous practice. Our results suggest this protocol can be used to reliably attain and maintain therapeutic vancomycin levels in patients receiving haemodialysis. Weight-based loading ensures attainment of target vancomycin levels. By delivering a fixed second dose and subsequent doses based on levels from previous dialysis sessions, the difficulty of availability of trough vancomycin level within the same dialysis session is avoided. Further to this, employing a nurse-led protocoled dosing regimen assured appropriate dosing of vancomycin ensued. At face value, the data seemed to suggest benefit of weight-based loading, however statistical analysis yielded non-significant p-values. Physician-led, weight-based loading was followed in only 55.6% of cases. Postulated reasons include unavailability of patient weight, initial unfamiliarity with the protocol and insecurity regarding the large doses used in obese patients. Subsequently, physicians were re-educated on the protocol, which is now readily available on the hospital intranet and displayed in the Haemodialysis Unit.
Although our study demonstrates a significant improvement in the proportion of therapeutic trough vancomycin levels, it has a number of limitations. Firstly, our outcome measure of vancomycin trough levels is a surrogate for hard clinical endpoints. We chose this outcome as attainment of therapeutic levels correlates with better clinical outcomes, and the main aim of this study was to validate a drug-dosing protocol. This study demonstrates the efficacy of maintenance dosing of vancomycin in this fashion, a future prospective study using clinical endpoints such as rates of clearance of infection would be desirable. Secondly, full compliance with weight-based loading was not achieved, limiting the validity of our protocol. However, as it is unlikely loading dose affects trough levels beyond the second dose, it does not affect the validity of our maintenance dosing approach. Thirdly, our study is limited by the sample size, which may account for the lack of difference in patients who received weight-based loading compared to those who did not. Moreover, although the protocol loading dose may not have been accurately followed, heavier patients often received higher doses. There was therefore some correlation between weight and first dose, even if accuracy in protocol adherence had not been achieved. Fourthly, multiple haemodialysis techniques were utilised in the study population. As the relative ability to clear vancomycin may affect trough levels, with a higher liability for sub-therapeutic levels in those receiving haemodiafiltration, further study is required to determine if our protocol is equally effective with haemodialysis and haemodiafiltration.
Conflict of Interest:
All authors confirm they have no conflict of interest in relation to this study.
Correspondence:
HM Gunning, Trinity Health Kidney Centre, AMNCH, Tallaght, Dublin 24
Email: [email protected]
References
1. Allon M. Dialysis catheter-related bacteremia: treatment and prophylaxis. Am J Kidney Dis. 2004; 44:779-791.
2. Vandecasteele SJ, Boelaert JR, De Vriese AS. Staphylococcus aureus infections in haemodialysis: what a nephrologist should know. Clin J Am Soc Nephrol. 2009; 4:1388-400.
3.,Bloombergen WE, Port FK. (1996) Epidemiological perspective on infections in chronic dialysis patients. Adv Ren Replace Ther. 1996; 3:201-7.
4. Tokars JI, Miller ER, Stein G. New national surveillance system for hemodialysis-associated infections: initial results. Am J Infect Control. 2002; 30:288-295.
5. Henrich WL. Principle and Practice of Dialysis. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2012.
6. Chambers HF. Antimicrobial agents: protein synthesis inhibitors and miscellaneous antibacterial agents. In: Hard-man JG, Limbird LE, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. New York: McGraw-Hill; 2001. p. 1262-1264
7. Launay-Vacher V, Izzedine H, Mercadal L, Deray G. Clinical review: Use of vancomycin in haemodialysis patients. Crit Care. 2002; 6:313-316
8. Ashley C. The Renal Drug Handbook. 3rd ed. Oxford: Radcliffe Publishing; 2009.
9. Daugirdas JT, Ing TS, Blake PG. Handbook of Dialysis. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
10. Vandecasteele SJ, De Vriese AS. Recent changes in vancomycin use in renal failure. Kidney Int. 2010; 77:760-4
11. Forouzesh A, Moise PA, Sakoulas G. Vancomycin ototoxicity; a reevaluation in an era or increasing doses. Antimicrob Agents Chemother. 2009; 53:483-6
12.Vandecasteele SJ, De Vriese AS. Review: vancomycin dosing in patients on intermittent haemodialysis. Semin Dial. 2011; 24:50-5.
13. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009; 66:82-98.
14. Tenover FC, Moellering RC Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus . Clin Infect Dis. 2007; 44:1208-15.
15. Bravo JJ, Diaz A, Donado E, Tarrago J, Tato F, Romero R, Sanchez-Guisande D, Mardaras J. Behaviour of vancomycin with the new techniques in haemodialysis. Nefrologia.2005; 25:527-34.
16. Vandecasteele SJ, Bacquer B, Vriese S. Implementation of a Dose Calculator for Vancomycin to Achieve Target Trough Levels of 15-20ug/mL in Persons Undergoing Haemodialysis. Clin Infect Dis. 2011; 53: 124-9
17. Meyer CC, Calis KA. New haemodialysis membranes and vancomycin clearance. Am J Health Sys Pharm. 1995; 52:2794-6.
18. Barth RH, DeVincenzo N. Use of vancomycin in high-flux haemodialysis: experience with 130 courses of therapy. Kidney Int. 1996; 50:929-36.
19. DeSoi CA, Sahm DF, Umans JG. Vancomcyin elimination during high-flux haemodialysis:kinetic model and comparison of four membranes. Am J Kidney Dis. 1992; 20:354-60.
20.Ariano RE, Fine A, Sitar DS, Rexrode S, Zelenitsky SA. Adequacy of a vancomycin dosing regimen in patients receiving high-flux haemodialysis. Am J Kidney Dis. 2005; 46:681-7
21. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984; 25:433-7.
22. Lindholm DD, Murray JS. Persistence of vancomycin in the blood during renal failure and its treatment by haemodialysis. N Engl J Med. 1966; 274:1047-51.
23. Lanese DM, Alfrey PS, Molitoris BA. Markedly increased clearance of vancomycin during haemodialysis using polysulfone dialyzers. Kidney Int. 1989; 35:1409-12.
24. Fresenius SE and Co., Bad Hamburg, Germany
25. Azzazy HM, Chou PP, Tsushima JH, Troxil S, Gordon M, Avers RJ, Chiappetta E, Duh SH, Christenson RH. Abbott AxSYM Vancomycin Assay: Multicentre Evaluation and Inference Studies. Ther Drug Monit. 1998; 20(2):202-8
P465