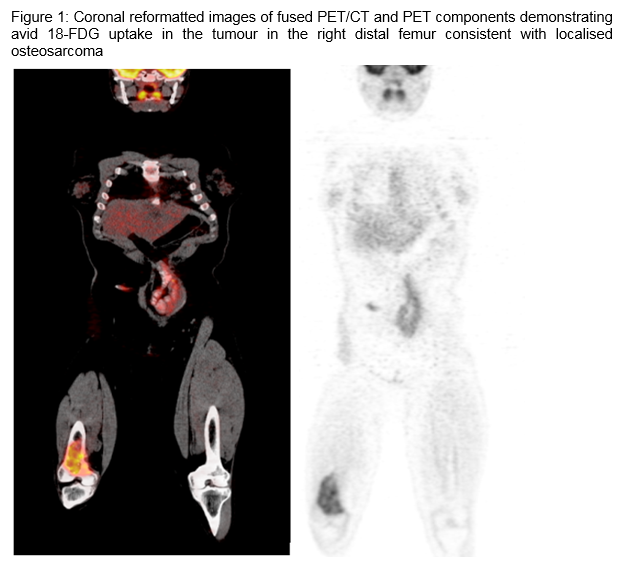
Osteosarcoma in a Patient Receiving Long-Term TNF Inhibitor Therapy
J Greene, GM O'Kane, E Aherne, GC O'Toole, DN Carney
Medical Oncology Department, Mater Misericordiae University Hospital, Dublin 7
Abstract
Patients with inflammatory bowel disease (IBD) have an increased risk of developing malignancy. The use of immunosuppressive therapies and tumour necrosis factor (TNF) inhibitors in these patients may provide a further risk for the development of malignancy. We report the clinical and pathological findings of a high grade osteosarcoma in a patient with Crohn’s disease receiving TNF inhibitor therapy. In this case, a 32-year old female presented with a painful right knee after receiving maintenance adalimumab for Crohn’s disease for a period of six years. There is a substantial hypothetical link between TNF inhibitor regimens such as adalimumab and an increased risk of malignancy. TNF inhibitor therapy should be ceased and chemotherapy and surgery is an effective combined modality approach in these patients. The role of TNF inhibitors in patients after cancer diagnosis is uncertain and further research is required to assess efficacy and safety.
Case Report
A 32 year old presented to the emergency department with a painful right knee. Her past medical history was significant for a 16-year history of Crohn’s disease. She was treated with courses of methotrexate and infliximab prior to commencing maintenance adalimumab six years prior to her presentation. She had no subsequent acute exacerbations of her Crohn’s disease while receiving adalimumab. Initial work up for her knee pain revealed a suspicious lytic area on X-Ray. A subsequent MRI showed an extensive destructive lesion of her distal femoral metaphysis through the lower third of the femoral shaft and distal femoral epiphysis with associated soft tissue mass swelling anteriorly. A right femoral biopsy was performed confirming high grade osteogenic sarcoma. PET CT confirmed an isolated lesion with no distant metastases. Adalimumab was discontinued. She was commenced on neoadjuvant chemotherapy with high dose methotrexate (8000mg/m2) alternating with doxorubicin (25mg/m2) and cisplatin (100mg/m2). Preoperatively PET CT revealed a radiological response to chemotherapy and reaffirmed the absence of distant disease. She underwent en bloc resection of the distal right distal femur. Final histology showed excellent response from chemotherapy with a grade III tumour necrosis of ninety five percent. Adalimumab remains on hold and she has had no acute exacerbations of her Crohn’s disease.
Discussion
Osteosarcomas are rare primary malignant tumours of bone that are characterized by the production of osteoid or immature bone by malignant cells1,2. Crohn’s disease is an autoimmune inflammatory condition that can affect any portion of the gastrointestinal tract. TNF-alpha is synthesized by activated macrophages and T cells as a transmembrane precursor protein and plays a pivotal role in causing chronic intestinal inflammation in inflammatory bowel disease (IBD)3,4. Synthetic TNF-alpha inhibitor antibodies have been shown to reduce this inflammatory process5. Adalimumab is a recombinant human monoclonal antibody directed against TNF that has been approved for use in Crohn’s disease. The activity of TNF-alpha against tumours in laboratory models suggested the possibility that inhibition of this cytokine might increase the clinical risk of malignancy. Proof of a link between TNF inhibitor use and the development of cancer in patients with IBD is difficult for several reasons due to the possible long term risk of malignancy from their underlying disease and previous immunosuppressive therapies. The potential relationship between TNF inhibitor use and the development of solid malignancies has been addressed in a number of case reports, reviews and meta-analyses and although some meta-analyses of clinical trial data have found an increased cancer risk with TNF inhibitor use, other observational data, particularly from registries, have not confirmed these findings6-9. Patients with IBD who are diagnosed with cancer should have their TNF inhibitor therapy held until their cancer is controlled10. Cytotoxic chemotherapy may also help control IBD during cancer therapy and corticosteroids may be used to control acute flares.
Though rare, there may be a link between TNF inhibitor therapy and malignancy including osteosarcoma as reported in this case. TNF inhibitor therapy should be ceased and chemotherapy and surgery is an effective combined modality approach in these patients. The role of TNF inhibitors in patients after cancer diagnosis is uncertain and further research is required to assess efficacy and safety.
Correspondence:
J Greene
Medical Oncology Department, Mater Misericordiae University Hospital, Dublin 7
Email: [email protected]
References
1. Sissons HA. The WHO classification of bone tumors. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 1976(54):104-8.
2. Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978-1997. Report from the Automated Childhood Cancer Information System project. European journal of cancer (Oxford, England : 1990). 2006;42:2124-35.
3. Ordas I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clinical pharmacology and therapeutics. 2012;91:635-46.
4. Nielsen OH, Ainsworth MA. Tumor Necrosis Factor Inhibitors for Inflammatory Bowel Disease. New England Journal of Medicine. 2013;369:754-62.
5. Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. The New England journal of medicine. 1999;340:1398-405.
6. Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiology and drug safety. 2011;20:119-30.
7. Mariette X, Matucci-Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R, Walsh C, Lawson R, Reynolds A, Emery P. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Annals of the rheumatic diseases. 2011;70:1895-904.
8. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA : the journal of the American Medical Association. 2006;295:2275-85.
9. Beaugerie L, Itzkowitz SH. Cancers Complicating Inflammatory Bowel Disease. New England Journal of Medicine. 2015;372:1441-52.
10. Bernheim O, Colombel JF, Ullman TA, Laharie D, Beaugerie L, Itzkowitz SH. The management of immunosuppression in patients with inflammatory bowel disease and cancer. Gut. 2013;62:1523-8.
p375