Patients safety incident reporting: the who, what, where, when and why?
DM Slattery
Department of Clinical Risk, State Claims Agency, Treasury Building, Grand Canal St., Dublin 2
Abstract
The landscape of patient safety is changing nationally and internationally. Recently, the number of preventable deaths in the USA annually is estimated at between 200,000-400,000.Nationally and internationally under-reporting of patient safety incidents is an opportunity for improvement. In 2015, 58.6% of patient related claims received by the State Claims Agency (SCA) had no previous patient safety incident reported, despite the legal obligation to report adverse events to the SCA. A detailed, national survey of the acute hospitals identified that there is significant variation in the modes and patterns of incident reporting, including variation in the percentage of incidents reported to the SCA, who decides what is reported, how it is reported, the backlog of incidents not reported and the time delay before reporting. This knowledge confirms that currently, comparisons between hospitals of equal activity are inaccurate. Reduction in variation and increased standardisation of patient safety incident reporting is required.
Introduction
In 1999 the Institute of Medicine’s, (USA) first quality report “To Err is Human: building a safer health system”1 identified preventable lapses in patient safety resulting in 44,000-88,000 American deaths each year, the eighth leading cause of death at the time. In 2002, the Joint Commission,(major accrediting body in the USA), identified six National Patient Safety Goals while the National Quality Forum listed 28 serious reportable events that should not happen. Subsequently a change in approach occurred: a payment system was used as a driver for patient safety with Centres for Medicare and Medicade Services stating they would not reimburse for certain conditions including wrong side/site surgery or hospital acquired infection. The Institute of Healthcare Improvement (IHI) published its global trigger tool (GTT) in 2000, which allowed healthcare services to track their own adverse events. Based on more modern studies published between 2008 and 2011, John James published an updated estimate of premature deaths associated with preventable harm: this figure is estimated at between 200,000-400,000 per year2. In 2003 in the UK, the National Reporting and Learning System, a central data base of patient safety incident reports was founded. It provides feedback to NHS organisations and publishes data freely on its website3 pertaining to comparative reporting rates of incidents, speed of reporting incidents, and incidents reported by degree of harm. Currently the same detail is not freely available in Ireland. Baseline data regarding the modes and patterns of incident reporting by acute hospitals in Ireland was important so that the effect of changes implemented may be measured.
Methods
A prospective, national survey of all acute hospitals, (n=50) as identified by the Acute Hospital Division of the Health Service Executive, was performed in the first half of 2015. The risk manager in each hospital was contacted directly by email and the purpose of the questionnaire explained. Completed questionnaires were returned to the clinical risk team at the SCA and results analysed.
Results
All acute hospitals responded. Variation and lack of standardisation was identified regarding the modes and patterns of incident reporting nationally by acute hospitals to the SCA. Forty nine (98%) acute hospitals responded that they reported incidents to the National Incident Management System (NIMS), but one hospital did not use NIMS. This has since been rectified. While the majority (n=38, 76%) of hospitals responded that they report to the SCA between 75-100% of incidents notified to them, 9 (18%) report 50% or less. Of these 9 (18%) hospitals, four report between 10-30% of incidents notified to them and 2 report less than 10%. Of the remaining 3 hospitals, one reported between 50- 75%, one did not report to NIMS, and one did not answer the question. Regarding a backlog of incidents not reported to NIMS, the majority (n=26, 52%) of hospitals responded that a backlog exists. The volume of the backlog, is less than 100 incidents in 12 (24%) hospitals, is between 100-500 in 8 (16%) and greater than 500 incidents in 6 (12%).
Regarding the time delay between the incident occurring and reporting it to NIMS, this was 1 month in the majority (n=34, 68%) of hospitals, 1-3 months in 8 (16%) and 6 months in 1 (2%) hospital. Regarding the others: 4 either “did not know” or said “it varies”, 2 did not answer the question, while 1 did not report to NIMS. Forty five (90%) hospitals responded correctly that it is a statutory obligation to report adverse events to the SCA while 5(10%) answered it is not. Thirty eight (76%) hospitals were aware that reporting incidents to an independent incident reporting system separate to NIMS did not fulfil the statutory obligation to report to the SCA, 6 (12%) responded that it did and 6(12%) did not answer the question. Five (10%) hospitals responded that they dual report to both NIMS and another independent incident reporting system e.g. Respond or Q Pulse. Regarding mode of reporting, 5 (10%) hospitals answered that they did not report directly to NIMS but sent all incident reports to an offsite location, Kilcreene Orthopaedic Hospital from where they were then reported to NIMS. When questioned regarding what incidents are reported to NIMS, 34 (68%) hospitals responded that they have a list to identify which incidents should be reported to the SCA. In 2/3 of hospitals (n=33, 66%) a quality safety/risk manager is involved in the decision as to what incidents are reported, in 11 (22%) all incidents are logged, while in the remaining hospitals, this decision is made by administrative staff (n=3, 6%), senior management (n=2, 4%), or director of nursing (n=1, 2%). In 40 (80%) hospitals an administrator entered the data on the computer, in 7 (14%) a risk manager did, while in 3 (6%) there was point of occurrence reporting for all staff.
Discussion
This is the first report in the literature outlining patterns of patient safety incident reporting nationally. Significant variation was identified in the modes and patterns of incident reporting by acute hospitals in Ireland to the SCA. Hospitals should report incidents using the NIMS, which is free and fulfils the legal obligation to report adverse events to the SCA. Dual reporting is inefficient. Incidents which are not reported, cannot be identified or prevented. Under-reporting is a national and international opportunity for improvement. In 2015, 58.6% of new patient claims received by the SCA nationally had no previous incident reported. While it is not always possible to know when an adverse event has occurred (e.g. a fistula may develop over time after surgery), and some claims may not be pursued by the plaintiff, there is room for improvement on the aforementioned figure. Internationally, a recent paper from the United States Department of Health identified that hospital staff did not report 86% of events to incident reporting system 4. Reporting incidents in a timely manner is critical to identify trends and patterns early, so that risk management recommendations and interventions can be made swiftly to prevent potential injury to patients. Sending incident reports to an off site location is inefficient:direct reporting from hospitals to NIMS is recommended.
Good quality reporting is key as evidenced by the recent "Clinical incident and claims report in maternity and Gynaecology services a five year review 2010-2014"5 which identified mis-categorisation of some incidents rated as “extreme in severity“ by the maternity services, when no actual injury had occurred. The current lack of standardisation of modes and patterns of incident reporting between hospitals makes comparisons inaccurate. Traditionally, efforts to identify adverse events have concentrated on voluntary reporting and tracking of errors. However, public health researchers have established that only 10-20% of errors are ever reported and of those 90-95% cause no harm to patients6. The IHI GTT uses a list of known adverse event “triggers” or “clues” to detect a greater number of adverse events. Applying this to adverse drug events led to the identification of one adverse drug event out of 4 admissions in a Belgian hospital,7 a prevalence of 16.4% adverse events/100 ICU days over 13 ICUs and 1,294 charts in Wisconsin, USA8 and 29.39 adverse events per 100 admissions, in a Turkish University hospital over a 1 year period9. High incident reporting across a range of severities of injury by a healthcare service, is generally associated nationally and internationally with a strong patient safety culture.
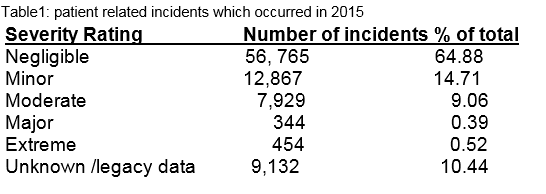
Over the last decade the number of incidents reported to the SCA has increased. During 2015, 87,491 patient related (including clinical and non clinical) incidents occurred, of which 0.52% (n=454) were rated extreme in severity, 0.39% (n=344) major, 9.06% (7,929) moderate, 14.71% (12,867) minor, 64.88% ( 56,765) negligible and 10.44% (9,132) unknown severity (Table 1). Through a combination of detailed analysis of incidents, their causes and contributing factors, identification of patterns and trends, open disclosure and engagement and empowerment of patients and their families, risk management recommendations and interventions may be made, to prevent potential harm to our patients.
Correspondence:
DM Slattery
Department of Clinical Risk, State Claims Agency, Treasury Building, Grand Canal St., Dublin 2
References
1. Kohn LT, Corrigan JM, Donaldson MS. Institute of Medicine. To err is human: building a safer health system, Washington DC : National Academy Press, 2000.
2. James JT. A new, evidenced–based estimate of patient harms associated with hospital care. J Patient Saf 2013; 3:122-8.
3. NRLS Available from https://report.nrls.nhs.uk/nrlsreporting/
4. Hospital incident reporting systems do not capture most patients harm. Levinson Dr Washington DC: US Department of Health and Human Services. Offices of the Inspectors General; 2012 January, report no OEI-O6-09-00091
5. Slattery D. Clinical incidents and claims report in Maternity and Gynaecology services:a five year review, 2010-2014. Available from http://stateclaims.ie/wp-content/uploads/2015/10/SCAClinicalIncidentsClaimsReportOct2015FINAL.pdf
6. Carnevali L, Krug B, Amant F, Van Pee D, Gerard V, de Bethune X, Spinewine A. Performance of the adverse drug event trigger tool and the global trigger tool for identifying adverse drug events: experience in a Belgian hospital. Ann Pharmacother 2013; 47:1414-9.
7. Resar RK, Rozich JD, Simmonds T, Haraden CR. A trigger tool to identify adverse events in the intensive care unit. Jt Comm J Qual Patiens Saf 2006; 32: 585-90.
8. Kurutkan MN, Usta E, Orhan F, Simsekler MC. Application of the IHI Global trigger tool in measuring the adverse event rate in a Turkish healthcare setting. Int J Risk Saf Med 2015; 27: 11-21.
p377