Mycobacterium avium complex pulmonary disease: characteristics and treatment in an Irish patient cohort.
EP Judge, M Ahmed, M Fitzgibbon, AM McLaughlin, J Keane
Department of Respiratory Medicine and Irish Mycobacteria Reference Laboratory, St James’s Hospital, James’s St, Dublin 8
Abstract
The prevalence of Mycobacterium avium complex (MAC) pulmonary disease is increasing globally. However, reliable national and international data relating to its epidemiology and management is lacking. During the period 2003-2014, MAC was isolated from the pulmonary samples of 75 patients at the Irish Mycobacteria Reference Laboratory (IMRL). Most patients (42, 56%) had underlying pulmonary disease, and 37 (49%) had clinical/radiographic characteristics consistent with MAC pulmonary disease. However, only 18 patients (24%) fulfilled internationally accepted criteria for diagnosis/treatment of this disease. Treatment was started in 13 (72%) of these cases, which is similar to internationally published treatment rates. The diagnosis of significant MAC pulmonary disease can be difficult, and treatment is not always warranted even when diagnostic criteria are met.
Introduction
Nontuberculous mycobacteria (NTM) are environmental organisms found in soil and water that are potential pulmonary pathogens in susceptible individuals. Published data suggest that Mycobacterium avium complex (MAC) is the most common cause of NTM pulmonary disease1,2. MAC encompasses several species of NTM including M. avium and M. intracellulare. The number of MAC isolates appears to be increasing internationally3,4. However, as NTM/MAC disease is non-notifiable in most countries (including Ireland), reliable estimates of its epidemiology and management are lacking. Furthermore, identification of MAC in pulmonary clinical samples frequently poses a significant diagnostic dilemma as it may represent contamination, colonisation or infection3. According to American Thoracic Society (ATS) guidelines, patients are classified as having NTM/MAC pulmonary disease if they meet specific microbiological, clinical and radiological criteria3. Despite this guideline document, it is likely that considerable variability (drug treatment versus no drug treatment) exists amongst clinicians in their management of MAC pulmonary disease2. The aim of this study was therefore to evaluate patient characteristics and management (according to ATS guidelines) of MAC pulmonary infection in an Irish hospital population.
Methods
All MAC isolates cultured at the IMRL from pulmonary samples obtained for TB analysis from patients of St. James’s Hospital, Dublin from 2003-2014 were identified using the GenoType Mycobacterium CM line probe assay (HAIN LifeScience, Nehren, Germany). Patient demographic and clinical data relating to each isolate was retrospectively collected from patient records. Radiological abnormalities consistent with NTM pulmonary disease were recorded from radiology reports. MAC isolate information was recorded from microbiology reports. Each patient was evaluated using ATS diagnostic criteria for NTM pulmonary disease based on collected information. Clinical management was recorded in each case.
Results
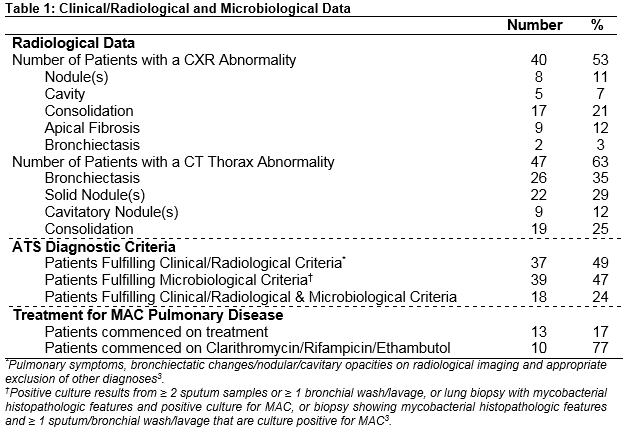
During the study period, 28,500 pulmonary samples (12,958 patients) were analysed for TB. There were 99 MAC isolates (75 patients) cultured from these samples (no cystic fibrosis cases, 52% male). Median age at time of culture positivity was 64 years and 52% of patients were current/former smokers. Most patients (56%) had underlying pulmonary disease (COPD (33%), bronchiectasis (25%)), and 30% of patients were immunosuppressed (21% HIV positive). Radiological and microbiological data are presented in Table 1. M. avium (79%) was the most commonly cultured organism (M. intracellulare 21%). Only 18 patients (24%) fulfilled ATS criteria for treatment of NTM. Treatment was commenced in 13 patients, and patients who received no treatment were clinically stable during follow-up. Reasons for not treating diagnosed patients included patient preference, clinical/radiological improvement over time and deranged liver function. Most patients (77%) were treated with the recommended regimen of clarithromycin, rifampicin and ethambutol.
Discussion
This study has shown that MAC is not commonly isolated from respiratory samples in an Irish hospital population. In addition, the majority of culture positive patients do not meet internationally-accepted criteria for NTM pulmonary disease, and treatment is only considered following fulfilment of these criteria and then physician/patient consensus. Two previous Irish studies suggest that MAC is the most common pulmonary NTM pathogen with an increasing prevalence in those with underlying pulmonary disease5,6. In contrast to previous studies, this study details how microbiological criteria for MAC disease treatment are not usually fulfilled following isolation from sputum/bronchoalveolar lavage. In addition, clinical/radiological criteria may not be fulfilled even when microbiological criteria are met. Therefore, a diagnosis of treatable MAC pulmonary disease was ultimately made in only a minority (24%) of MAC isolate patients, which is similar to internationally published figures7.
A recent pan-European study suggests that there is significant heterogeneity in NTM pulmonary disease treatment practices according to specific country and severity of symptoms2. In this study, we have shown that treatment for MAC pulmonary disease is only started in an Irish hospital cohort when internationally accepted clinical/radiological and microbiological criteria for this disease are fulfilled. In addition, treatment was only started in 13 (72%) of suitable cases and non-treated patients remained clinically stable during follow-up. This therefore illustrates how it is often reasonable not to start treatment in selected patients. This figure also compares with reported treatment rates of 43-68% in Europe, USA and Asia8-10. We consequently believe that patients with NTM pulmonary disease are preferably managed by physicians with suitable expertise in centres where they can be enrolled in prospective epidemiological/clinical trials in order to enhance our understanding of this condition.
Correspondence:
Prof. Joseph Keane
Department of Respiratory Medicine, St. James’s Hospital, Dublin 8
Email: [email protected]
References
1.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, Beylis N, Boeree MJ, Cacho J, Chihota V, Chimara E, Churchyard G, Cias R, Daza R, Daley CL, Dekhuijzen PN, Domingo D, Drobniewski F, Esteban J, Fauville-Dufaux M, Folkvardsen DB, Gibbons N, Gomez-Mampaso E, Gonzalez R, Hoffmann H, Hsueh PR, Indra A, Jaqielski T, Jamieson F, Jankovic M, Jong E, Keane J, Koh WJ, Lange B, Leao S, Macedo R, Mannsaker T, Marras TK, Mauqein J, Milburn HJ, Mlinko T, Morcillo N, Morimoto K, Papaventsis D, Palenque E, Paez-Pena M, Piersimoni C, Polanova M, Rastoqi N, Richter E, Ruiz-Serrano MJ, Silva A, da Silva MP, Simsek H, van Soolingen D, Szabo N, Thomson R, Tortola Fernandez T, Tortoli E, Totten SE, Tyrrell G, Vasankari T, Villar M, Walkiewicz R, Winthrop KL, Wagner D. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013 Dec;42:1604-13.
2. Wagner D, van Ingen J, Adjemian J, Lange C, Prevots DR, Griffith D, Gallagher J, Gupta R, Haworth C. Annual prevalence and treatment estimates of nontuberculous mycobacterial pulmonary disease in Europe: A NTM-NET collaborative study. ERJ September 1 2014;44 Suppl 58 P1067.
3. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007 Feb 15;175:367-416.
4. Moore JE, Kruijshaar ME, Ormerod LP, Drobniewski F, Abubakar I. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995-2006. BMC Public Health 2010;10:612.
5.Kennedy MP, O’Connor TM, Ryan C, Sheehan S, Cryan B, Bredin C. Nontuberculous mycobacteria: incidence in Southwest Ireland from 1987 to 2000. Respir Med 2003 Mar;97:257-63
6. Chong SG, Kent BD, Fitzgerald S, McDonnell TJ. Pulmonary non-tuberculous mycobacteria in a general respiratory population. Ir Med J 2014 Jul-Aug;107:207-9.
7. van Ingen J, Bendien SA, de Lange WCM, Hoefsloot W, Dekhuijzen PN, Boeree MJ, van Soolingen D. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax Jun 2009;64:502-6.
8.van Ingen J, Adjemian J, Morimoto K, Wagner D, Lange C, Haworth C, Gallagher J, Prevots R, Gupta R, Griffith D. Comparison of treatment practices for nontuberculous mycobacterial pulmonary disease in Japan, Europe and United States. ERJ September 1 2014;44 Suppl 58 P1067.
9. Koh WJ, Hong G, Kim SY, Jeong BH, Park HY, Jeon K, Kwon OJ, Lee SH, Kim CK, Shin SJ. Treatment of refractory Mycobacterium avium complex lung disease with a moxifloxacin-containing regimen. Antimicrob Agents Chemother 2013 May;57:2281-5.
10. G, Lee KS, Moon JW, Koh WJ, Jeong BH, Jeong YJ, Kim HJ, Woo S. Nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Natural course on serial computed tomographic scans. Ann Am Thorac Soc 2013 Aug 10:299-306.
P396