A Cost Minimisation Analysis of NOACs Compared to Warfarin to Attain Therapeutic Anticoagulation amongst AF Patients, Pre- and Post- Cardioversion
O’Shea M.1, Murphy, A1, Kirby A.1, Allen G.2, O'Shea S.2, Curtin R.2
1Department of Economics, Cork University Business School, University College Cork
2Cork University Hospital, Wilton, Cork
Abstract
Atrial fibrillation can be managed with anticoagulation and restoration of normal sinus rhythm using direct current cardioversion (DCCV). To reduce the risk of thromboembolism, anticoagulation pre-and-post DCCV is recommended. This study investigates the cost effectiveness of using NOACs compared to warfarin to attain therapeutic anticoagulation amongst AF patients pre-and-post DCCV. Propensity score matching revealed no statistically significant difference in outcomes from using NOACs and Warfarin. A cost minimisation study was performed; demonstrating a cost differential of €209 between those administered NOACs and warfarin pre- and post-DCCV. This study demonstrates how using NOACs compared to warfarin to attain therapeutic anticoagulation amongst AF patients pre-and-post DCCV is cost effective.
Introduction
Atrial fibrillation (AF) affects approximately 1-2% of the population worldwide and is a major cause of stroke1, which is a major contributor to disability and death. AF is managed using anticoagulants (warfarin or new oral anticoagulants (NOACs)) and restoration of normal sinus rhythm (NSR) using direct current cardioversion procedure (DCCV). The latter is an outpatients procedure whereby synchronized, low-voltage electrical shocks are administered to the heart to restore a NSR2. Evidence demonstrates that DCCV is effective and safe, with success in over 90% of cases whereby NSR is restored3,4. Nevertheless, less than half of patients remain in NSR after one year3 4. Factors which influence success and maintenance of NSR have been investigated and include duration of AF, cardiac size and function, rheumatic heart disease, significant mitral valve disease, delays in conversion, left atrial enlargement, and older age3. Furthermore, therapeutic anticoagulation for 3-4 weeks prior to DCCV is recommended to reduce the risk of thromboembolism3,5, from 3-5%5-8, to less than 1%3,9,10. The traditional anticoagulant prescribed is warfarin, and while this is effective in reducing the risk of thromboembolism it has been found to be unpredictable, thus requiring monitoring and adjustments in dosage11 which can contribute to delays in performing DCCV. This can have a negative impact on the success of DCCV and maintenance of NSR in the longer term3. NOACs have emerged as safe and effective alternatives to warfarin [12-14], eliminating the need for monitoring and dose adjustments3. While these advantages of NOACs make them attractive for patients and clinicians, their high drug cost relative to warfarin means their reimbursement in public health care systems may be difficult. This study aims to determine the cost impact of using NOACs compared to warfarin to attain therapeutic anticoagulation for patients with AF pre- and post-DCCV.
Ireland is chosen as a case study for this cost minimisation analyses as it represents a typical public health care system with a substantial reimbursement system for pharmaceutical drugs15,16. It is expected that by 2050 the population aged over 65 years will double to 1.4 million17. While, somewhat behind its neighbours, Ireland has the opportunity to implement cost effective policies and initiatives when managing services for the silver economy. Furthermore, while several NOACs (Apixaban, Dabigatran and Rivaroxaban) have been deemed cost effective in Ireland by the National Centre of Pharmacoeconomics (NCPE), budget implications and concerns over safety have hindered their use18-20.
Methods
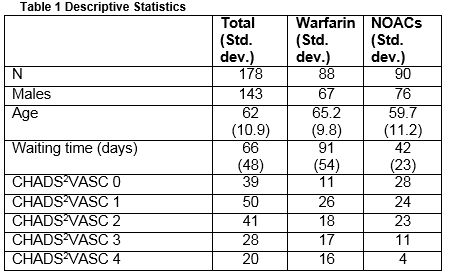
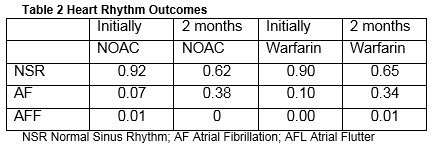
Evidence from the Cardiology Department at Cork University Hospital was employed where there is an advanced nurse practitioner-led DCCV service. CUH acts as a regional centre for secondary and tertiary care for the catchment population of 550,000 served by the HSE Southern area, and a supra-regional centre for a total a population of 1.1 million21. A sample of 178 patients was derived from a registry in the Cardiology Department at CUH over two six-month periods between 2013 and 2014. No patient in the study had to have either Warfarin or NOAC discontinued for an emergency situation. This is a rare occurrence in real life with both NOACs and Warfarin and would be unlikely to have an impact on costs. In the sample, 80% were male with an average age of 62 years (std. dev. 10.9). The minimum CHADS2VASC Score was 0 and the maximum was 4 with modal category of 1. NSR was achieved in 91% of cases (8% remained in AF and 1% moved to atrial flutter (AFF)). The average waiting time to receive cardioversion was 66 days (std. dev. 48). In the sample, 88 patients were administered warfarin and 90 received a NOAC (76 received dabigatran and 14 rivaroxaban). Amongst those receiving warfarin, 67 were male with an average age of 65.2 years (std. dev. 9.85). The modal CHADS2VASC score was 1 and 58% of the sample had CHADS2VASC score greater than or equal to 2. Post DCCV, NSR was achieved in 90% of cases and the average waiting time was 91 days for patients prescribed warfarin (std. dev. 54). After two months, only 65% remained in NSR. In the NOAC sample there were 76 males and 15 females. The average age in that sample was 59.7 years (std. dev. 11.2). The modal CHADS2VASC score was 0 and 42% of patients had a CHADS2VASC scores greater than or equal to 2. NSR was achieved in 92% of cases and the average waiting time for DCCV was 42 days (std. dev. 23). After two months, only 62% of patients remained in NSR.
A cost minimisation analysis (CMA) was undertaken to reveal the costs associated with warfarin compared to the NOACs for DCCV for AF patients. A CMA is a type of economic evaluation wherein two or more interventions are compared22. Here the administering of NOACs is compared to the administering of warfarin pre- and post-DCCV. A key assumption of such an evaluation is that the outcomes of the different programmes are broadly equivalent22, thus only the programme which delivers the outcome at the least cost is preferred. To conduct the CMA there were two stages. Firstly, to determine there was no expected difference in outcome (i.e. in achieving NSR after DCCV), between those administered warfarin and NOACs, propensity score matching was performed. The nearest neighbour-matching method was employed to estimate standard errors to determine if the difference in outcome (i.e. achieving NSR) is statistically different between the two treatments. Secondly, a micro-costing study was performed to reveal the costs associated with using NOACs compared to warfarin for patients with AF pre- and post-DCCV. This involved identifying, measuring and valuing the associated resource use. To identify and measure the costs, an observation study was performed and expert opinion was elicited in CUH in January 2014. To value the costs, secondary data from previous studies was used where available; public sector salary scales were used to value staff costs and overheads as per HIQA recommendations23; and drug costs were sourced from the Primary Care Reimbursement Service (PCRS) and published sources.
Results
Propensity score matching was performed to determine whether there was no expected difference in outcome (i.e. in achieving NSR after DCCV) between those administered warfarin and NOACs. Using nearest neighbour-matching method the standard errors indicated that the difference in outcome (i.e. achieving NSR) is not statistically different between those receiving warfarin and NOACs immediately post DCCV (n treatment =90; n control = 42; std error = 0.080; test statistic -0.069). Maintaining NSR two months post DCCV was also assessed. Again the propensity score matching found no clinical difference between warfarin and NOACs with respect to achieving and maintaining NSR after two months (n treatment =90; n control = 42; std error = 0.115; test statistic 0.296).
Having determined that there was no statistically significant difference in outcomes after DCCV between those for whom therapeutic anticoagulation was achieved with warfarin and NOACs, a CMA was performed to indicate which programme is the most cost effective.
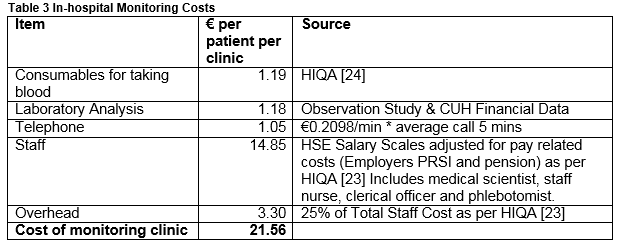
A micro-costing study was performed to reveal the costs associated with anticoagulation between people on warfarin and NOACs receiving DCCV. This involved identifying, measuring and valuing the associated resource use. The costs were categorised into monitoring costs and drug costs. Monitoring costs applied to warfarin only and included consumables for testing INR levels (nitrile gloves, sterile cotton wool balls, injection swabs, needle, monovette and tape), laboratory analysis (staff costs, innovin, cuvettes, and analyser tubes), telephone communications (relaying results), staff costs (clerical, nurses, phlebotomy) and overheads. Resources were measured using a combination of primary data (observation study, CUH January 2014), and secondary data24. Following HIQA23 guidelines resources were valued using market values and HSE salary scales.
The monitoring clinic costs per patient per visit consist of taking blood €1.19 (as per HIQA24); laboratory analysis €1.18; telephone costs €1.05; staff €14.85 and overheads €3.30. Thus the total cost of the monitoring clinic per patient per visit is €21.56. (This is comparable with the results found in another hospital based Warfarin monitoring clinic in Ireland25). Given that the average wait for DCCV is 90.829 days, which is equivalent to 12.98 weekly visits. At €21.56 per visit, the cost of monitoring prior to DCCV for those administered warfarin is €279.76.
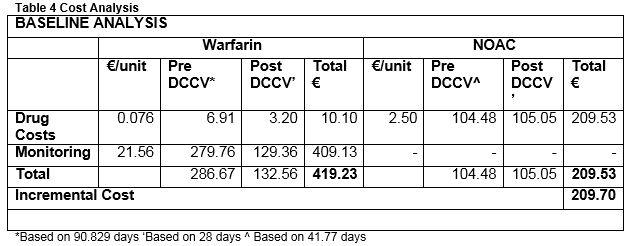
The cost of pharmaceutical drug costs was sourced from the Medicines Management Programme review of oral anticoagulants for stroke prevention with AF20. The price per day for dabigatran is €2.54 (150mg and 110 mg) and rivaroxaban is €2.29 (20mg and 15 mg). The cost of warfarin was sourced from Barry18 as €0.076 per day (adjusted for inflation).
Evidence from the registry revealed average (weighted) waiting time for NOAC patients waiting for DCCV is 41.77 days and weighted cost is €2.50 per day. Thus the average pharmaceutical drug costs associated with NOACs prior to DCCV is €104.48. The average pharmaceutical drug cost per patient for warfarin (90.829 day wait) is €6.91.
Patients receiving DCCV should remain on anticoagulants up to six weeks post procedure. This amounts to additional pharmaceutical costs of €105.05 for patients administered NOACs. For patients adminstered warfarin, the pharmaceutical cost is €3.20, and the monitoring costs €129.36 (6 visits). Thus the total cost for those administered NOACs for anticoagulation before and after DCCV is €209.53 and for warfarin is €419.23. The incremental cost is €209.70. Administering NOACs being almost 50% less expensive than warfarin when weekly monitoring and pharmaceutical drugs costs are accounted for.
Discussion
Having matched the samples from the registry data, there was no statistically significant difference in achieving NSR between those administered warfarin and NOACs. With respect to costs, the average costs for those administered NOACs are €209 less than for those administered warfarin pre- and post-DCCV. This is despite the daily drug costs for NOACs being 32 times more expensive than warfarin. However, the waiting time for DCCV is less amongst those administered NOACs compared to warfarin, and there are no monitoring requirements – monitoring costs represent 98% of the costs associated with those administered warfarin.
There are limitations to this study which centre on the data employed, particularly pertaining to sample size and lack of randomisation. In addition, the authors are cognisant of the variability in the results owing to the real world nature of the data. However, should waiting times be improved upon it would reduce the cost associated with DCCV when administering NOACs further.
This analysis considers patients who were not previously administered an anticoagulant. Given the demographics of the patient population it is likely that patients with a higher risk of stroke owing to AF may require long term anticoagulation post DCCV. Long term cost effectiveness of NOACs compared to warfarin has been investigated by decision makers on a country-by-country basis. This analysis revealed that administering NOACs pre and post a DCCV can reduce costs relative to administering warfarin without impacting outcomes; thus can be considered cost-effective.
Since the data was collected, new guidance on preferred prescribing has emerged in Ireland. Despite three NOACs being considered cost effective, warfarin remains the first line therapy for stroke prevention in AF in Ireland20. Apixaban is the first line option when warfarin is not considered suitable owing to an allergy or labile INR levels20. As populations age and AF becomes more prevalent, patient demands for the latest drug or technology increase thus placing increasing pressures on the delivery of health care. Ensuring the right patient receives the right treatment in a cost-effective manner continues to be a challenge. This study demonstrates how economic evaluations can be used to inform resource allocation decisions in clinical settings.
Conflict of Interest
The authors have read and understood IMJ policy on declaration of interests and declare that they have no competing interests
Correspondence:
Dr Ann Kirby, Department of Economics, Cork University Business School, University College Cork, Cork
Email: [email protected]
REFERENCES
1. Lichten, C., S. Castle-Clarke, C. Manville, V. Horvath, E. Robin, J. Krapels, S. Parks, M. Sim, O. van Zijverden, and J. Chataway, The future of anticoagulation management in atrial fibrillation in Europe. 2015.
2. NIH. What is Cardioversion? Explore Cardioversion 2012 [cited 2015 08/07/2015]; Available from: http://www.nhlbi.nih.gov/health/health-topics/topics/crv.
3. Abu‐El‐Haija, B. and M.C. Giudici, Predictors of Long‐term Maintenance of Normal Sinus Rhythm After Successful Electrical Cardioversion. Clinical cardiology, 2014. 37: p. 381-385.
4. Van Gelder, I.C., H.J. Crijns, W.H. Van Gilst, R. Verwer, and K.I. Lie, Prediction of uneventful cardioversion and maintenance of sinus rhythm from direct-current electrical cardioversion of chronic atrial fibrillation and flutter. The American journal of cardiology, 1991. 68: p. 41-46.
5. Coleman, C.M., S. Khalaf, S. Mould, O. Wazni, M. Kanj, W. Saliba, and D. Cantillon, Novel Oral Anticoagulants for DC Cardioversion Procedures: Utilization and Clinical Outcomes Compared with Warfarin. Pacing and Clinical Electrophysiology, 2015.
6. Bjerkelund, C.J. and O.M. Orning, The efficacy of anticoagulant therapy in preventing embolism related to DC electrical conversion of atrial fibrillation. The American journal of cardiology, 1969. 23: p. 208-216.
7. January, C., L. Wann, and J. Alpert, AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: executive summary [published online March 28, 2014.]. J Am Coll Cardiol, 2014.
8. Morris, J.J., Y. Kong, W.C. North, and H.D. McIntosh, Experience with “cardioversion” of atrial fibrillation and flutter. The American journal of cardiology, 1964. 14: p. 94-100.
9. Gentile, F., A. Elhendy, B.K. Khandheria, J.B. Seward, C.M. Lohse, W.-K. Shen, K.R. Bailey, S.C. Montgomery, K.N. Burger, and A.J. Tajik. Safety of electrical cardioversion in patients with atrial fibrillation. in Mayo Clinic Proceedings. 2002. Elsevier.
10. Pritchett, E.L., Management of atrial fibrillation. New England Journal of Medicine, 1992. 326: p. 1264-1271.
11. Menzin, J., L. Boulanger, O. Hauch, M. Friedman, C.B. Marple, G. Wygant, J.S. Hurley, S. Pezzella, and S. Kaatz, Quality of anticoagulation control and costs of monitoring warfarin therapy among patients with atrial fibrillation in clinic settings: a multi-site managed-care study. Annals of Pharmacotherapy, 2005. 39: p. 446-451.
12. Flaker, G., R.D. Lopes, S.M. Al-Khatib, A.G. Hermosillo, S.H. Hohnloser, B. Tinga, J. Zhu, P. Mohan, D. Garcia, and J. Bartunek, Efficacy and safety of apixaban in patients after cardioversion for atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation). Journal of the American College of Cardiology, 2014. 63: p. 1082-1087.
13. Piccini, J.P., S.R. Stevens, Y. Lokhnygina, M.R. Patel, J.L. Halperin, D.E. Singer, G.J. Hankey, W. Hacke, R.C. Becker, and C.C. Nessel, Outcomes after cardioversion and atrial fibrillation ablation in patients treated with rivaroxaban and warfarin in the ROCKET AF trial. Journal of the American College of Cardiology, 2013. 61: p. 1998-2006.
14. Yadlapati, A., C. Groh, and R. Passman, Safety of short-term use of dabigatran or rivaroxaban for direct-current cardioversion in patients with atrial fibrillation and atrial flutter. The American journal of cardiology, 2014. 113: p. 1362-1363.
15. Barry, M., L. Tilson, and M. Ryan, Pricing and reimbursement of drugs in Ireland. The European Journal of Health Economics, formerly: HEPAC, 2004. 5: p. 190-194.
16. Barry, M. and L. Tilson, Recent developments in pricing and reimbursement of medicines in Ireland. Expert review of pharmacoeconomics & outcomes research, 2007. 7: p. 605-611.
17. CSO, Population and Labour Force Projections 2011-2041, 2008, Central Statistics Office: Stationery Office, Dublin, Ireland.
18. Barry, M., CORRESPONDENCE: Dabigatran versus Warfarin in Patients with Atrial Fibrillation. New England Journal of Medicine, 2009. 361: p. 2671-2675.
19. McCullagh, L. and M. Barry, Prescribing trends for dabigatran etexilate in primary care. Irish medical journal, 2012.20. MMP, Oral anticoagulatns for stroke prevention in non-valvular atrial fribrillation in Preferred Drugs2015, Medicines Management Programme.
20. CUH. Profile of Cork University Hospital. 2015 08/07/2015]; Available from: http://www.cuh.hse.ie/.
21. Drummond, M.F., M.J. Sculpher, G.W. Torrance, B.J. O'Brien, and G.L. Stoddart, Methods for the economic evaluation of health care programmes2005: Oxford university press.
22. HIQA. Guidance on Budget Impact Analysis of Health Technologies in Ireland. 2015 16 July 2015; Available from: http://www.hiqa.ie/healthcare/health-technology-assessment/guidelines.
23. HIQA, Economic evaluation of repeat universal antenatal screening for HIV in the third trimester of pregnancy, 2012, Health Information and Quality Authority: Dublin, Ireland.
24. Walsh, C., A. Murphy, A. Kirby, and C. Vaughan, Retrospective costing of warfarin. Irish medical journal, 2014.
P480