A role for susceptibility weighted imaging in progressive multifocal leukoencephalopathy
Yap SM1, Murray B1, Lynch T1, Kavanagh E2, MacMahon P2
1Department of Neurology, Mater Misericordiae University Hospital, Dublin 7, Ireland
2Department of Radiology, Mater Misericordiae University Hospital, Dublin 7, Ireland
Abstract
We report a radiologic finding on magnetic resonance imaging (MRI) of the brain of two cases of progressive multifocal leukoencephalopathy (PML) of hypointense signal of subcortical U-fibres on susceptibility weighted (SW) sequence. The first case is a 50-year-old man recently treated with chemotherapy including rituximab for non-Hodgkin's lymphoma. The second case is a 64-year-old woman with human immunodeficiency virus (HIV) infection. Iron deposition is a likely causative factor. We propose that SWI may be especially useful in the assessment of indeterminate cases to reduce the likelihood of a missed diagnosis of PML.
Introduction
Progressive multifocal leukoencephalopathy (PML) is a severe demyelinating disease of the brain resulting from reactivation of JC virus in the setting of immunosuppression. We report two cases of PML with hypointense signal of subcortical U-fibres on susceptibility-weighted (SW) sequence of magnetic resonance imaging (MRI) of the brain. We propose that SWI may be of diagnostic value in PML.
Case 1
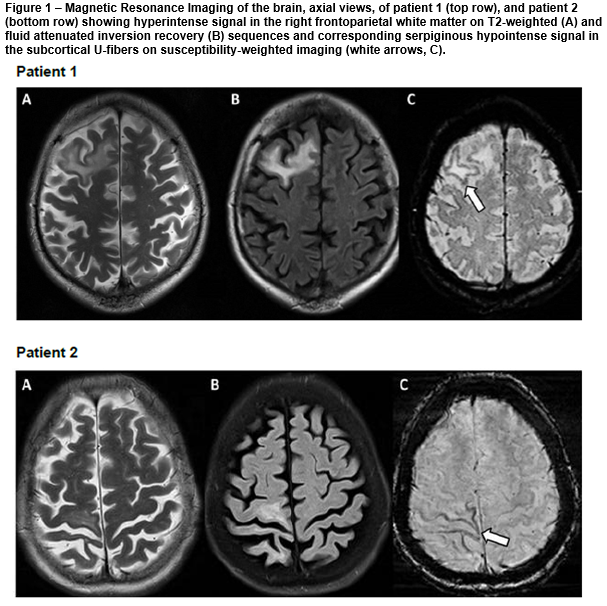
A 50-year-old man was admitted into hospital with a three-month history of fatigue, slurred speech and poor balance. Examination showed apathy, word-finding difficulties and ataxia. 21 months prior, he was diagnosed with stage III non-Hodgkin’s lymphoma and treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) chemotherapy for six months followed by maintenance rituximab. Initial MRI brain showed confluent areas of high signal on T2 and fluid attenuated inversion recovery (FLAIR) sequences in the right frontal lobe, periventricular white matter, right thalamus and left midbrain with diffusion restriction. Cerebrospinal fluid (CSF) JC virus DNA was positive. PML was confirmed and he was treated with plasma exchange. Repeat MRI showed enlarging T2 and FLAIR hyperintensities in the right frontoparietal white matter with serpiginous hypointense SWI signal affecting the subcortical U-fibres [figure, top row, A-C]. He was treated with intravenous immunoglobulin. The patient improved clinically and subsequent MRI brain showed stable findings.
Case 2
A 64-year-old HIV-positive woman was admitted into hospital with cough, weight loss and worsening left hemiparesis. She was initially diagnosed with stroke at another hospital two months prior whereby MRI brain reported restricted diffusion in the right frontoparietal lobe. Interval MRI brain showed progression of signal hyperintensity in the right frontoparietal region on DWI, T2 and FLAIR sequences with corresponding hypointense SWI signal change affecting subcortical U-fibres [figure, bottom row, A-C]. She was discharged after treatment for pneumonia and presumed stroke. Four weeks later, the patient presented with worsening left hemiparesis. CSF JC virus DNA was positive. Repeat MRI brain showed more extensive lesions with patchy peripheral enhancement. A diagnosis was made of PML and immune reconstitution inflammatory syndrome (IRIS). She developed severe pneumonia and succumbed to her illness.
Discussion
We present one case of rituximab and chemotherapy-associated PML and one case of HIV-associated PML-IRIS, both demonstrating low intensity SWI signal of subcortical U-fibres. These SWI abnormalities have been reported in natalizumab-associated PML in patients with multiple sclerosis1. The American Association of Neurology (AAN) diagnostic criteria for PML proposed in 2013 do not include SWI abnormalities2. Diagnosing PML may prove difficult due to a wide range of differential diagnoses including cerebral lymphoma, glioma, ischaemia, vasculitis, chemotherapy-induced leukoencephalopathy and opportunistic infection.
SWI provides a sensitive technique for detecting deoxyhaemoglobin, haemosiderin, ferritin, calcium and air3. Quantitative susceptibility mapping (QSM) allows for differentiation of diamagnetic substances e.g. calcification from paramagnetic ones e.g. iron deposition and blood degradation products4. Mechanisms underlying SWI abnormalities in PML are unclear. One prospective case series demonstrated SWI hypointensities in U-fibres with corresponding hyperintensities on QSM, hence showing a paramagnetic effect4. Iron deposition is a likely causative factor1,4,5. Calcification is unlikely given lack of corresponding hyperattenuation on CT5. The serpiginous rim of hypointense SWI signal is not typical of haemorrhage.
The histopathologic triad in PML includes multifocal demyelination, enlarged oligodendroglial nuclei and enlarged bizarre astrocytes2. JC virions are commonly detected in the nuclei of infected oligodendrocytes2. It was reported that oligodendrocytes are the predominant iron-containing cells in the brain and are abundant within white matter tracts6. Cellular injury may lead to release of free iron which generates reactive oxygen species that causes oxidative cell death7
In conclusion, we propose that SWI may be included in routine MR imaging protocols for the assessment of possible PML. However, the sensitivity and specificity of this sign have yet to be formally studied. This finding may prove especially useful where CSF JC virus result and / or clinical features may be equivocal, hence reducing the likelihood of a missed diagnosis of PML.
Correspondence
Siew Mei Yap, Department of Neurology, Mater Misericordiae University Hospital, Dublin 7, Ireland. [email protected]
Conflict of Interest
All authors report no conflicts of interest in the preparation of this case series.
References
1. Hodel J, Outteryck O, Verclytte S, Deramecourt V, Lacour A, Pruvo JP, Vermersch P, Leclerc X. Brain magnetic susceptibility changes in patients with natalizumab-associated progressive multifocal leukoencephalopathy. AJNR Am J Neuroradiol. Epub 2015 Aug 27.
2. Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Seivar JJ, Bartt R, Major EO, Nath A. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013 Apr 9;80:1430-8.
3. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009 Jan;30:19-30.
4. Carra-Dalliere C, Menjot de Champfleur N, Deverdun J, Ayrignac X, Nerrant E, Makinson A, Casanova ML, Labauge P. Use of quantitative susceptibility mapping (QSM) in progressive multifocal leukoencephalopathy. J Neuroradiol. Epub 2015 Oct 13
5. Miyagawa M, Maeda M, Umino M, Kagawa K, Nakamichi K, Sakuma H, Tomimoto H. Low signal intensity in U-fiber identified by susceptibility-weighted imaging in two cases of progressive multifocal leukoencephalopathy. J Neurol Sci. 2014 Sep 15;344:198-202.
6. Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia. 1996 Jun;17:83-93.
7. Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007 Jul;4:371-86.
(P549)