Antenatal Pertussis Vaccination: Why are General Practitioners Reluctant? A Mixed Methods Study
A O’Connell1, A Tummon1, K Coleman1, A Jordan1, J McCormack2, M E Kelly2, 3
1GP Registrar, Western Training Programme in General Practice, Galway University Hospital, Galway, Ireland
2Assistant Programme Director, Western Training Programme in General Practice, Galway University Hospital, Galway, Ireland
3Lecturer in the Discipline of General Practice, Medical School, National University of Ireland, Galway
Abstract
Pertussis has a disproportionately higher morbidity and mortality in infants less than 3 months of age. International and national guidelines recommend pertussis vaccination during pregnancy, as a safe and effective way to protect these infants. Antenatal pertussis vaccination uptake rates remain suboptimal, with many health care professionals (HCPs) still not recommending it. The reasons underlying this reluctance on behalf of HCPs have not been fully established. This study aims to evaluate the current practice and attitudes of General Practitioners (GPs) with regard to antenatal pertussis vaccination. An embedded mixed method design was used. The response rate was 41% (n=109). 54% of GPs who responded (n=59) routinely recommend antenatal pertussis vaccination. Safety concerns and a sense of isolation emerged as the major qualitative themes. More safety data, adequate funding from the Health Service Executive (HSE) and support from secondary care may help to increase the GP recommendation rate and enhance vaccination uptake in pregnancy
Introduction
Pertussis is a highly contagious respiratory tract infection. The illness is particularly severe in children under 3 months of age, with a reported case fatality rate in this age group of up to 4%1. Despite universal infant and childhood immunization, pertussis in infants remains a major public health problem worldwide2. During the 2012 pertussis outbreak in Ireland, 458 pertussis notifications were reported to the Health Protection Surveillance Centre (HPSC)3. Three infant deaths occurred and all of these cases were infants less than two months old. These infants were too young to benefit from the Primary Immunisation Schedule in Ireland, which begins at 2 months of age4. It has been shown that vaccination of women during pregnancy protects the infant prior to their first vaccination. Antenatal vaccination is thought to be protective in this period through both passive antibody transfer and reduced exposure to maternal pertussis infection5,6. Routine antenatal pertussis vaccination was introduced in the United Kingdom in 2012. Since then, there has been a 91% reduction in pertussis cases in infants whose mothers were vaccinated compared to those who were not6. In Ireland, from 2011-2012, there were 37 notified cases of pertussis in infants. In 20 of these cases (54%), information on maternal antenatal vaccination status was reported. None of the mothers of the affected infants had been vaccinated during the antenatal period3.
The safety of pertussis immunisation in pregnancy using the Tetanus, diphtheria and acellular pertussis (Tdap) vaccination has been widely studied and has not been shown to harm the foetus5,7. In 2013, the National Immunisation Advisory Committee (NIAC) advised all GPs in Ireland to recommend pertussis vaccination during each pregnancy, between 27 and 36 weeks gestation8, in line with international recommendations2. Despite these recommendations, international uptake rates remain suboptimal6,9. In Ireland, there is no national recording of uptake rates of pertussis vaccination during pregnancy. However, in 2013, a “point of care audit” reported that just 6.4% of mothers had received the pertussis vaccine during pregnancy. Furthermore, 69.9% of women who received the pertussis vaccine cited their GP as their only source of information10. Studies have shown that the most effective way to increase the uptake of vaccinations in pregnancy remains direct recommendation from a HCP11,12. However, many HCPs still do not recommend antenatal vaccination12. The reasons underlying this reluctance on the part of HCPs and GPs have not been established. Therefore the aim of this study was to evaluate the current practices and attitudes of GPs in the West of Ireland with regard to antenatal pertussis vaccination.
Methods
An embedded mixed methods design was used, with equal priority given to both quantitative and qualitative strands of data13. As a literature review did not identify any suitable previously validated questionnaire; a novel study instrument was designed, piloted with three GPs, amended accordingly and distributed. It comprised a two page anonymous questionnaire. Five-point Likert scales and dichotomous questions were used to obtain quantitative data, while open-ended and free comment questions were used to obtain qualitative responses. All GPs in Galway, Mayo and Roscommon who were registered in the Irish Medical Directory 2015 – 2016 were invited to participate. The three GPs who were involved in piloting the questionnaire were excluded. The anonymous questionnaire, a cover letter, participant information leaflet, and consent form were posted to GPs. A reminder letter was sent two weeks later and a maximum of three weeks was allowed from first dispatch to receipt of the self-completed questionnaire.
As completed questionnaires were returned they were ascribed a numerical code – e.g.GP1 being the first received questionnaire. Excel was used to analyse the quantitative data. Results were described using means, percentage responses and Chi Square (χ²) with significance level based on a p-value of 0.05. The qualitative information was transcribed into a Microsoft Word document and then coded and analysed thematically, by the four principle investigators (AJ, AO’C, AT, KC), according to Braun and Clarke’s six stages of thematic analysis14. Ethical approval was granted by the Irish College of General Practitioners’ (ICGP) Research Ethics Committee. Funding was awarded by the ICGP Research and Education Foundation Grant.
Figure 1. Questionnaire (appendix 1)
Results
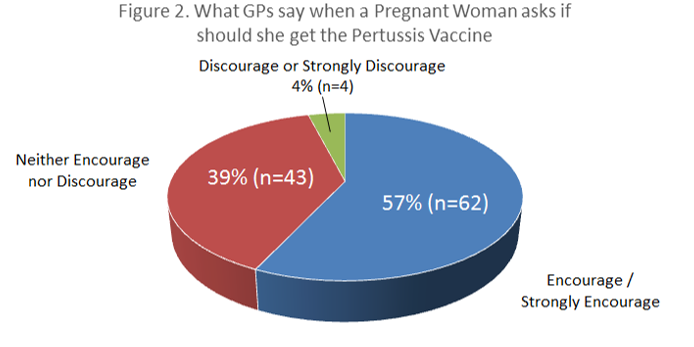
A total of 268 questionnaires were distributed, with 109 completed questionnaires returned within the study window, giving a response rate of 41%. There were 59 (55%) female respondents and 49 (45%) male respondents. One respondent did not specify a gender. Fifty-four percent of GPs who responded (n=59) routinely recommend the pertussis vaccine to pregnant women. Female GPs were even more likely to recommend it with 64% (37/58) of female GP respondents recommending pertussis vaccination in pregnancy versus 44% (21/48) of male GP respondents (χ² = 4.26, p<0.05). Just four percent of GPs surveyed (n=4) would actually discourage or strongly discourage the vaccine. Fifty-seven percent of GP respondents (n=62) would encourage or strongly encourage the vaccine, while 39% (n=43) would allow the woman to make her own decision.
Safety Concerns
Eighty-one percent (n=48/59) of GPs who did not recommend the vaccine have safety concerns compared to 29% (n=14/48) of GPs who did recommend the vaccine (χ² = 41.80, p <0.001). Among the GPs who do not routinely recommend antenatal pertussis vaccination, 83% (N=40/48) were concerned that a woman might link an adverse event in pregnancy with the vaccine, while 65% (N=31/48) felt that more safety data was needed before they could recommend the vaccine with confidence. Less than 20% of GP respondents felt that women were declining this vaccine because of financial concerns. Safety concerns also emerged as the dominant theme on qualitative analysis. There were concerns about possible future complications from pertussis vaccination in pregnancy and respondents felt that “more safety data is required” (GP47). Fear of litigation was very palpable - “I am always concerned that in any pregnancy complications may come back to haunt me” (GP44). As vaccinations are not licensed in pregnancy several GPs were fearful of liability in the event of any adverse outcome - “It is not licensed for that use, so in the event of a reaction occurring we are not supported” (GP 46). Some GPs found it very difficult to go “against the strongly held belief to avoid all medications in pregnancy” (GP107). Yet other GPs were prepared to embrace the new guidelines to avoid the “possible devastating consequences of pertussis in a neonate” (GP100). Several GPs emphasised that it was “her own choice” (GP 88), in an effort to balance the need to promote a HSE recommendation with their own personal reservations - “Will give information and let the patient decide. Wouldn’t initiate conversation re pertussis” (GP 6).
GPs’ Sense of Isolation
GPs’ “sense of isolation” emerged as the second major theme. GPs frequently reported feeling a lack of support from the HSE - “I don’t feel I’ve gotten a huge amount of information on this from the HSE” (GP60). As part of the Special Type Consultation (STC) Scheme for GMS patients, a GP can claim for the administration of influenza vaccination in pregnancy but not pertussis. This led to GPs questioning whether pertussis antenatal vaccination has full “government approval and support” (GP52). Some GPs also expressed a perception of lack of support from their colleagues in secondary care - “Consultant Obstetricians’ attitudes towards pertussis vaccination have been at best passive” (GP104).
Facilitators to Help Increase Uptake
The questionnaire included possible initiatives, based on the literature, which may help increase uptake. 80% (n=87) of GPs surveyed agreed that further education would be helpful – “Maybe a local paediatric and public health doctor to talk to us at CME”. (GP95) and 87% (n=95) were in support of a national media campaign - “Better publicity on television/radio/papers...” (GP58). 86% (n=94) agreed that the inclusion of a reminder in the National Maternity Notes would encourage women to ask about the vaccine and reinforce that the vaccine is recommended by the HSE - “Place in maternity chart” (GP62). 93% (n=102) felt that GPs should be reimbursed for giving this vaccine - “HSE is happy to reimburse GPs for administrating flu vaccine to pregnant women with medical card but not the pertussis vaccine – where’s the logic??” (GP104).
Discussion
This is the first Irish study to ascertain the current practice and attitudes of GPs to pertussis vaccination in pregnancy. The study found that 54% (n=59) of GPs surveyed are routinely recommending antenatal pertussis vaccination. A UK study found that only 34% of women were offered the vaccine at their GP practice and only 24% of women reported a “meaningful discussion with their GP about it”15. Safety concerns were shown to be a major factor influencing a GP’s decision whether or not to recommend the vaccine. This is comparable to several studies which have shown that for many HCPs the thought of vaccinating pregnant woman remains a taboo16, 17. Further longitudinal safety data may be required before GPs can feel confident in recommending the vaccine in pregnancy. Furthermore, no vaccine is licensed for use during pregnancy, it is therefore understandable that GPs may have fears of litigation. This was discussed in a recent review which emphasised that although there is a lack of pre – licensure studies in pregnant women this “does not preclude its use in pregnancy”16. Vaccination labelling needs to be amended, in line with national and international guidelines. Safety concerns also led several GPs in our study to adopt a passive approach, with 39% of respondents neither encouraging nor discouraging the vaccine. This was also emphasised in the qualitative data, with several GP respondents reluctant to initiate the conversation about pertussis vaccination. This is consistent with another study which reported that GPs were reluctant to provide a strong opinion or recommendation to their patients. Instead, they let the patient decide for themselves17. A structured educational programme and support for HCPs may reduce physician uncertainty and empower them to provide a strong, positive approach to vaccination.
The second theme that emerged from this study was a sense of isolation as numerous GPs felt unsupported by the HSE and their secondary care colleagues. This is consistent with literature elsewhere which recommended more support from governments and health authorities18. It has been shown that incorporation of maternal vaccinations into standard antenatal documentation leads to improved vaccine uptake in pregnancy19. Participants in this study also saw merit in this approach. A similar UK study concluded that vaccines in pregnancy should form part of national antenatal care guidelines and should be recommended at the first and subsequent antenatal visits15. The number of GP respondents recommending pertussis vaccination was higher than anticipated and higher than UK data15. However, our study limitations may include participant bias, as GPs with a special interest in obstetrics or pertussis vaccination may have been more likely to respond. A further targeted mailing to non responders may have helped to enhance the response rate. Furthermore, this study collected data from GPs in Galway, Roscommon and Mayo and so may not represent the views of GPs nationally. Conversely, a two page anonymous questionnaire was thought to be the most apt method of data collection in order to maximise the participation rate and minimise participant inconvenience. In addition, an embedded mixed methods design allowed for the collection of rich information which could not have been gained from a purely quantitative study.
In conclusion, this study found that the majority of GPs surveyed are currently recommending antenatal pertussis vaccination. Future research involving pregnant women and practice nurses, using a similar study instrument, would complement this study and help to inform public health. More support from secondary care and the HSE, in addition to more prospective studies on the safety of the vaccine, are required in order to increase GP recommendation rates.
Declaration of Interest
None to declare
Acknowledgements
The programme directors and our GP trainers on the Western Training Programme in General Practice.
All GPs who participated in this study.
Correspondence: Adrian Jordan, Western Training Programme in General Practice, The Nurses Home, Galway University Hospital, Newcastle, Galway, Ireland
E-mail: [email protected]
References
1. National Immunisation Advisory Committee. National Immunisation Guidelines - Pertussis. Dublin: 2016. http://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter15.pdf
2. Advisory Committee on Immunisation Practices. Updated recommendations for the use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine in pregnant women. Morbidity and Mortality Weekly Report (MMWR) 2013; 62(7): 131-135.
3. Cotter S, Gee S. Pertussis and burden of disease in infants - missed opportunities for immunisation. HPSC Disease Surveillance Report 2015; 16(5).
4. Health Protection Surveillance Centre. Pertussis in Ireland - emerging trends. http://www.hpsc.ie/A-Z/VaccinePreventable/PertussisWhoopingCough/SurveillanceReports/
5. Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, Walter EB, Jackson LA, Englund JA, Edwards MS, Healy CM, Petrie CR, Ferreira J, Goll JB, Baker CJ. Safety and Immunogenicity of Tetanus Diphtheria and Acellular Pertussis (Tdap) Immunization during Pregnancy in Mothers and Infants: A Randomized Clinical Trial. JAMA 2014; 311(17): 1760–1769.
6. Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry N, Miller E, Ramsay M. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet 2014; 384: 1521 – 1528.
7. Donegan K, King B, Byran P. Safety of pertussis vaccination in pregnant women in the UK - an observational study. BMJ 2014; 349(g): 4219.
8. Health Service Executive. Whooping cough/Pertussis letter to GPs. http://www.hse.ie/eng/health/immunisation/hcpinfo/correspondence/
9. Cherry JD. Tetanus-diphtheria-pertussis immunization in pregnant women and the prevention of pertussis in young infants. Clinical Infectious Diseases 2015; 60(3): 338-340.
10. O'Lorcain P, Cotter S, O'Flanagan D. Point of Care Audit: Pertussis (Whooping Cough) and seasonal influenza vaccination during pregnancy 2012-2013 in Maternity Units in Ireland. Health Protection Surveillance Centre 2015.
11. Yuen CY, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women – a systematic review. Vaccine 2014; 32(36): 4602-4613.
12. Taksdal SE, Mak DB, Joyce S, Tomlin S, Carcione D, Armstrong PK, Effler PV. Predictors of uptake on influenza vaccination – a survey of pregnant women in Western Australia. Australian Family Physician 2013; 42(8): 582 – 586.
13. Cresswell JW, Clark VP. Writing and evaluating mixed methods research. Designing and conducting mixed methods research, 2nd ed. California, USA: SAGE Publications, Inc.; 2011.
14. Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology 2006; 3: 77-101.
15. Donaldson B, Jain P, Holder BS, Lindsay B, Regan L, Kampmann B. What determines uptake of pertussis vaccine in pregnancy? A cross sectional survey in an ethnically diverse population of pregnant women in London. Vaccine 2015; 33: 5822–5828.
16. de Martino M. Dismantling the Taboo against Vaccines in Pregnancy. International Journal of Molecular Sciences 2016; 17: 894.
17. Maher L, Dawson A, Wiley K, Hope K, Torvaldsen S, Lawrence G, Conaty S. Influenza vaccination during pregnancy: a qualitative study of the knowledge, attitudes, beliefs, and practices of general practitioners in Central and South-Western Sydney. BMC Family Practice 2014, 15:102
18. Yaqub O, Castle-Clarke S, Sevdalis N, Chataway J. Attitudes to vaccination: A critical review. Social Science & Medicine 2014; 112: 1-11.
19. Webb H, Street J, Marshall H. Incorporating immunizations into routine obstetric care to facilitate Health Care Practitioners in implementing maternal immunization recommendations. Human Vaccines and Immunotherapeutics 2014; 10(4): 1114-1121.
(P634)