Consent for routine neonatal procedures: A study of practices in Irish neonatal units. How do we compare with the gold standard BAPM guidelines?
M A Ryan, C A Ryan, E Dempsey, R O’Connell
Department of Neonatology, Cork University Maternity Hospital, Wilton, Cork
Abstract
The Irish National Consent Policy (NCP)¹ proposes that the legal requirement for consent extends to all forms of interventions, investigations and treatment, carried out on or behalf of the Health Service Executive (HSE). This study employs a quantitative descriptive approach to investigate the practices for obtaining consent for an identified group of routine neonatal procedures in neonatal facilities throughout Ireland. The BAPM (British Association of Perinatal Medicine)² guidelines were identified as ‘gold standard’ for the purposes of this study. The results indicated a lack of consistency between participating units pertaining to the modes of consent utilised and notable variances from ‘gold standard’ guidelines. Unanimity was evident for 3 procedures only (administering BCG, 6-in-1, and donor breast milk to infant). Significant findings related to EEG with video recordings, MRI/CT and gastro intestinal imaging, screening of an infant with suspected substance abuse or retinopathy of prematurity screening (ROP), administration of Vitamin K, and the carrying out of a lumbar puncture.
Introduction
Consent may be implicit/ - inferred/deducible by the infant’s presence in the unit, or explicit -clearly stated in detail which is easily understandable, and may require the support of a signature. This study explores the current practices for a group of 25 randomly chosen routine neonatal procedures. Informed consent is required before any form of medical treatmentᵌ. It may be impractical to seek consent for each and every intervention within the neonatal environs but parents are the designated proxy decision makers for the infant, with parental input/ understanding of all actions and how they are in the infants ‘best interest’ required. Informed consent is the ideal benchmark against which the ethical and legal organizational practices must be measured. Two way discussions are the cornerstone of the informed consent process, which underpins an individual’s (or parents) ability to exercise his/her autonomy in relation to his/her (infant’s) medical treatment. The person providing a particular health and social care service is ultimately responsible for ensuring that the service user is consenting to what is being done¹. Documenting the discussion that took place, any questions asked, as well as the patient’s decision to consent or refuse the proposed treatment is an essential component of the informed consent process.
Method
A literature search was undertaken, and in the absence of a valid instrument, a survey questionnaire was designed in co-ordination with expert practitioners. A small pilot study was conducted to identify any potential issues and amended accordingly. An initial ‘Pre-*notification survey’ letter was sent to all participants to enhance awareness and increase the response rate to the survey. This was followed 10 days later by the survey itself. Each invited participant was asked to respond by a closed tick box system, if consent for each procedure was assumed/implicit, explicitly sought verbally or was explicit consent sought with the support of a signature. A response rate of 90% was achieved. There are 19 maternity hospitals with the equivalent 19 neonatal units of varying levels and capacity. Each unit was invited to participate along with two paediatric hospitals with specialist neonatal facilities. Ethical approval was obtained individually from all institutions whilst consent of access obtained from all but one site (N=20). The clinical nurse manager (CNM) in each unit was identified as the target participant and considered to be in the best position to provide the information required. The survey was voluntary and anonymous.
Results
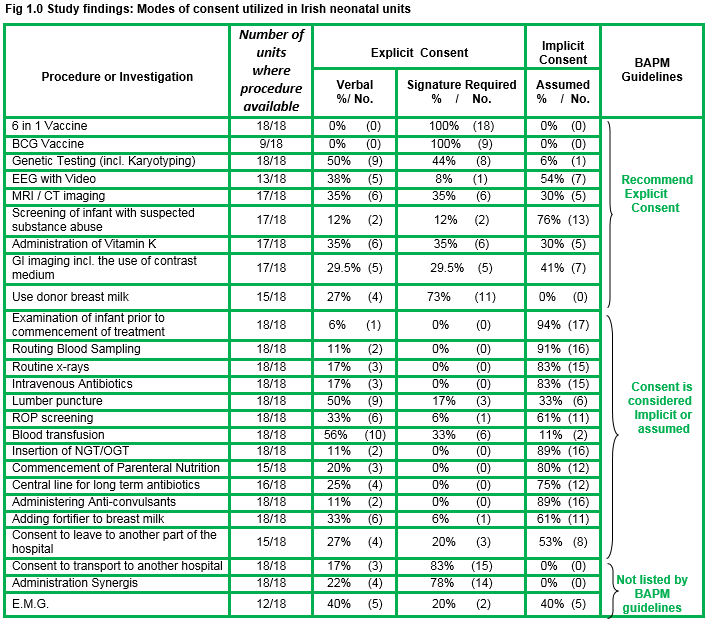
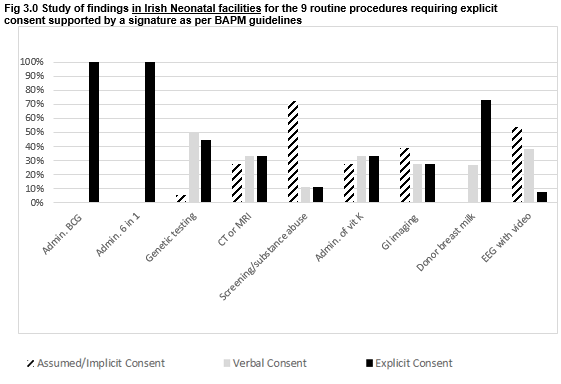
The response rate to the questionnaire was 90% (18/20 neonatal facilities).There was universal agreement in seeking explicit consent for three procedures: BCG vaccination, 6-in-1 vaccination and administering donor expressed breast milk (EBM-pooled, pasteurised breast milk from an accredited milk bank), with the support of a signature not sought in 25% of cases for the latter.
Significant findings related to disparities between units and BAPM guidelines pertaining to the routine procedures of electroencephalogram (EEG) with video recording, screening of an infant for suspected substance abuse, CT/MRI imaging, ROP screening, administration of Vitamin K to a term infant, gastrointestinal imaging with contrast, lumbar puncture, and consent to go to another part of hospital.
Testing for toxicology should not be undertaken without consent as per BAPM guidelines. We need to consider if it is acceptable, that over three quarters of responding facilities(76%) in Ireland assume consent to send the blood/urine of a newborn with a suspected drug addicted mother for drug screening.
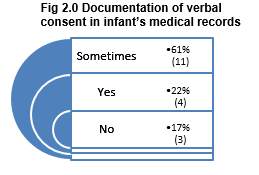
Results indicated that verbal consent was poorly documented with 78% or respondents reporting ‘sometimes’ or ‘no’ to documentation of consent in the infant’s medical notes. Consideration if ‘sometimes’ is a reference to some procedures or to some individual’s documentation practices, suggests a potential for bias in this study. Although it must be acknowledged that differences in practices are evident between facilities.
Discussion
Arguably, practices utilised for adults should be effective for proxy decision makers (parents or guardian) of a newborn infant in a nonemergency situation. The National Institute for Health and Care Excellence (NICE guidelines) support the requirement for informed explicit consent for lumbar puncture⁵ which is documented in patient’s records and incorporates analgesia given prior/ during the procedure⁶. Conflicting local practices are evident in this study with an assumption of consent in one third of participating units. Screening for ROP is a relatively low risk procedure in premature infants but has been known to have short term effects on the cardiac and respiratory function⁷. In the UK supreme court, Lord Scarman invited medical and legal representatives to reconsider the duty of a doctor towards a patient and a decision to consent to treatment (or medical investigation) did not entirely rest on medical considerations but also to the importance attached by patients (or in this case parent’s)⁸. International guidelines in obtaining informed consent prior to screening for ‘Retinopathy of Prematurity’ (ROP) propose oral and written information pertaining to the screening process incorporating the disclosure of undesirable risks and the potential adverse effects their infant’s systemic status⁹. Clinical Nurse Managers’ response in this survey demonstrated a support for an assumption of consent.
Technology and research findings in the area of genetics and human genome appear to be ahead of ethical/legal guidelines. Genetic testing can help to diagnose an illness or help to predict the development of an illness in the future. The Medical Council recommend counselling about the possible consequences of genetic testing before consent is sought¹⁰. Issues pertaining to the future use of such samples, and implications of findings for infant and family must be discussed with parents prior to analysis. There is a question as to the ethic and legality of ‘assuming’ consent for screening of an infant with suspected substance abuse. Testing for toxicology on the guise of ‘best interest’ or ‘duty of care’ to the infant with questionable implications of the results on medical treatment, does appear to lay the burden of proof for the decision to carry out such screening on the clinician involved ¹¹.
Parental permission for diagnostic imaging such as CT (Computerised Tomography), MRI (Magnetic Resonance Imaging) and GI (gastro intestinal) Imaging with contrast medium does not comply with ‘gold standard’ guidelines. Looking to international guidelines explicit consent is advisable for which incorporates verbal / written information with the support of a signature in some cases¹². It has now become routine that EEG monitoring is accompanied by video surveillance to allow for visual identification of neurological events should they occur. Parental consent is paramount in obtaining video surveillance or photographs of an infant as part of any investigation or treatment of a condition¹³,¹⁴. Irrespective of motive, it is inappropriate to assume consent for such an investigation. Foreign-language translations of consent forms, recruiting materials and other study materials must always be available.
The National Advisory Committee on Bioethics recently published its opinion on ‘consent to blood transfusions’. This opinion reflected the culmination and deliberation of data from the National and International arena and the committee itself. It proposes that there is a need for greater consistency and transparency in how the consent process for blood transfusion is conducted across the health care system and that a system of specific informed consent for blood transfusion represents an ethically appropriate and proportionate measure to achieve these objectives⁴. The NCP’s (2013) resonates a commitment to enhance the process of informed consent. What we need to ask ourselves - is the assumption of consent ever enough and is the term ‘assumed consent’ a permit to erode the concept of informed consent in neonatal care? The Medical Council (2016)³ supports the legal requirement of informed consent before the provision of any medical investigation, examination or treatment and an essential component of respect for patients’ autonomy. These principles and values are woven through the fabric of the – Partnership, Practice and Performance – frame work. Obtaining consent is not a one off event and requires ongoing dialogue/communication carried out in an open honest and comprehensive manner with parents.
In a non-emergency situation within the neonatal unit, questions need to be asked pertaining to the inappropriateness of leaning on the concept of ‘best interest’ or ‘duty of care’ without an attempt to obtain informed consent. Within the neonatal environs it may be considered an easy option to assume consent for procedures that parents have no knowledge about (nor are they aware they have a choice), or to steer vulnerable parents in one direction or another. Heavy workloads, scarcity of time, service user commitments, availability of a clinician (with adequate knowledge of the procedure) potentially compromises or challenges the components of informed consent. There is also a need to consider the potential human factor of ‘bias’ that will always exist in decisions with to regard to investigations or procedures.From a legal perspective the greatest pitfalls to informed consent occur due to inadequate or absent documentation of the complete details of discussions¹³. Should conflict and legal formalities arise pertaining to informed consent, judgment is frequently based on the recordings in the medical notes. Ad hoc practices pertaining to documentation were widely evident in this study. All nations have their own unique culture and Ireland is no exception. In making a comparative to the set ‘gold standard’ guidelines cognizance mus be given to the fact that these guidelines have their origin in another culture with different values. Health care operates in a dynamic environment and what was considered ‘gold standard’ in 2011 in the UK in reality may not be adaptable to Ireland in 2017.
Uniformity of consent practices in neonatal care can only be addressed through the establishment of national guidelines. Ireland must develop its own guidelines coherent to our culture, our National Consent Policy (2013), best practice and incorporating the viewpoints of all stakeholders. Scientific evidence is emerging daily as is evidence of ‘risk’ from / or relating to a procedure. Such guidelines will reduce inconsistencies in practice, confusion amongst service users/health care professionals, implicate standards of care along with our ethical and legal standings. These guidelines may be altered and reviewed to reflect latest research and Irish culture in its current time. Clinical audit, quality assurance, education and training are essential in providing safe and effective healthcare responding to the commitment by the National Consent Policy (2013), and the Medical Council (2016) to enhance the process of informed consent. It seems somewhat inappropriate that it is acceptable for variation to exist in practice. The National Standards for Safer and Better Health Care (2012)¹⁵, highlights the need to consider the underlying ethical principle of patient autonomy. These standards also acknowledge that we are not all practicing in accordance with the law and recognize a need to change.
Correspondence:
Mary Anne Ryan,
Irish Centre for foetal and Neonatal Research (INFANT Research Centre) Department of Neonatology,
Cork University Maternity Hospital / UCC Cork
Contact email: [email protected]
Conflicts of interest
None declared
Acknowledgement
I am indeed grateful to all Clinical Nurse Managers who took time to complete this study. Special thanks to Lucille Bradfield.
References
1. Health Services Executive. National Consent Policy. Health Service Executive: Stationary office, 2014. Available from: www.hse.ie
2. British Association of Perinatal Medicine (2011). Consent for Common neonatal investigations and treatments. www.bapm.org/publications/documents/guidelines/procedures.pdf
3. Irish Medical Council. Guide to Professional Conduct and Ethics for Registered Medical Practitioners, 8th Edition. Dublin(Ireland): Irish Medical Council; 2016
4. National Advisory Committee on Bioethics Ireland. Specific Informed Consent for Blood Transfusing: The Ethical Considerations. Dublin: Department of Health stationary office. 2013
health.gov.ie/wp-content/uploads/2014/07/SpecificConsent_Blood-Transfusion1.pdf
5. National Institute for Health Care Excellence. NICE Charter. Available from:www.nice.org.uk
6. Doherty,C.,Forbes. Diagnostic Lumbar Puncture. Ulster Medical Journal. 2014; 82 (2): 93-102.
7. Mitchell, A., Green, A.,Jeffs, D.,Robertson P. Physiological Effects of Retinopathy of prematurity screening examinations. Advances in Neonatal Care. 2011 Aug; 11(4): 291–297.
8. Montgomery v Lanarkshire Health Board [2015] UKSC 11. Available from https://www.supremecourt.uk/decided-cases/docs/UKSC_2013_0136_Judgment.pdf
9. Royal College of Paediatrics and Child Health, Royal College of Ophthalmologists, British Association of Perinatal Medicine & BLISS. UK Retinopathy of Prematurity Guideline. May 2008. Available from:
www.iapb.org/sites/iapb.org/files/RCO%20ROP%20guidelines%20all%202008.pdf
10. Irish Medical Council. Guide to Professional Conduct and Ethics for Registered Medical Practitioners, 8th Edition. Dublin(Ireland): Irish Medical Council; 2016
11. Holme, N., Raju, M., Griffiths, C., Pramod, S., Hardisty, A., Johnston, K. Urine Toxicology, is it simply a waste of resources? High Flow therapy usages regionally and locally. Arch Dis Child foetal and Neonatal Ed. 2014; 99(1):67.
12 Royal Australian and New Zealand College of Radiology: Medical Imaging Consent Guidelines. Available from: http://www.ranzcr.edu.au/
13 Madden,D. Medical Ethics and Law. Dublin: Bloomsbury Professional
14 Devakumar, D., Brotherton, H., Halbert, J., Clarke, A., Prost, A., Hall, J. Taking ethical photos of children for medical research purposes in low resource settings: An explorative qualitative study. Biomedical Central. 2013; 14(27): 14-27.
15 Health Information and Quality Authority HIQA. National Standards for Safer and Better Health Care. Dublin: HIQA. 2012.
https://www.hiqa.ie/system/files/Safer-Better-Healthcare-Standards.pdf
p584