Continuous Glucose Monitoring in the Management of Neonatal Hypoglycaemia
S.M. McGlacken-Byrne, R. O’Neill, A. Jenkinson, J.F.A. Murphy
Neonatal Intensive Care Unit, National Maternity Hospital, Holles St., Dublin, Ireland
Abstract
A quality improvement project was carried out in a Level 3 Neonatal Intensive Care Unit (NICU) which aimed to successfully implement the use of continuous glucose monitoring systems (CGMS) in hypoglycaemic infants. Piloting of the device revealed several potential practical barriers to its reliably successful implementation. Five Plan-Do-Study-Act (PDSA) cycles followed, tackling these problems and other issues inductively identified throughout the project. Parents and multi-professional stakeholders were involved and consulted throughout. Change was measured on a runchart using qualitative and quantitative feedback. Problem rate per patient was reduced to zero by the end of a one-month study period. This study used basic quality improvement methodologies to implement a change intervention in a structured manner and elucidated aspects of its use that need to be adapted for its successful incorporation into real-life clinical practice.
Introduction
We designed a quality improvement project successfully implement the use of continuous glucose monitoring systems (CGMS) in hypoglycaemic infants in a Level 3 Neonatal Intensive Care Unit (NICU). Evidence from empirical research studies suggests that CGMS are safe and have the potential to improve the management of neonatal hypoglycaemia1,2. CGMS was first piloted in our NICU in June 2017. A New Generation Enlite™ Sensor (Medtronic, Northridge, California) was inserted into a term baby admitted with hypoglycaemia using a previously described insertion technique3.
Five key problems were elucidated as potential barriers to the effective implementation of CGMS in our unit: lack of NICU staff confidence in device usage, infant discomfort during device removal, calibration errors, wireless connection disruptions during nursing cares, and bruising after device removal resulting in parental dissatisfaction. We reflected that while CGMS use in neonatal populations has been successfully implemented within the controlled environments of research studies, it may need to be adapted for use within resource-limited, time-constrained clinical practice. We aimed to reduce the problem rate per patient associated with CGMS use in our NICU from 5 to 0 over a one-month period.
Methods
The key stakeholders for this project were identified as patients, parents, nursing staff, medical staff, and clinical engineers. The principles of CGMS usage, its potential to improve neonatal hypoglycaemia management, and the challenges encountered during the pilot process were discussed at a multidisciplinary teaching session to educate and engage neonatal staff and to brainstorm the way forward. A driver diagram outlined the project’s aims (Graph 1). Ethical approval was obtained from the Ethics Committee of the National Maternity Hospital, Dublin. Written informed consent was obtained from all parents before participation. This study received no external funding and CGMS units were purchased by the hospital’s procurement department.
This study was conducted from June 2017 to July 2017 in a level 3 NICU. A CGMS was inserted into five consecutive infants admitted with hypoglycaemia (Table 1). A pilot process followed by five “Plan-Do-Study-Act” (PDSA) cycles tested the change intervention. A run chart tracked the improvement in problem rate per patient over time. The Neonatal Infant Pain Scale (NIPS) was used during this and all further cycles to monitor infant discomfort during insertion and removal of the sensor, with a score over three indicating discomfort (Graph 2).
Eligible for inclusion were term neonates ³1.5kg admitted for hypoglycaemia (<2.6mmol/L) within the first 48 hours of life. Hypoglycaemic infants were managed as per the local hospital protocol, which includes performing standard intermittent capillary glucose sampling pre-feeds three-hourly using Hemacue® Glucose 201 DM System (Radiometer, UK). In addition, a New Generation Enlite™ Sensor (Medtronic, Northridge, California) (10.5mm needle, 27GA gauge) was inserted into the lateral thigh at the time of admission guided by the insertion technique3. After a two-hour initialisation period, the sensor requested a point-of-care capillary glucose reading for calibration. Once calibrated, the sensor transmitted interstitial glucose readings to a Minimed® REAL-Time Transmitter and displayed an averaged glucose value every 5 minutes on a MiniMed® 530G System (both Medtronic, Northridge, California). Sensor readings are displayed in real-time, but for the purposes of our study these readings were only reviewed if the sensor alarmed to signify significant hypo- or hyperglycaemia. Thus, the standard settings of the sensor were left unchanged, and the sensor alarmed at BSL levels ≤2.2 or ³24.0mmol/L. Infants with hypoglycaemia outside of this range (i.e. infants with BSLs between 2.2mmol/L and 2.6mmol/L) were managed as per the local hospital protocol. The Enlite™ sensor requires input of calibration readings at least 12 hourly. Sensors in this study were calibrated more regularly as the three-hourly point-of-care BSLs taken for clinical management were also used for calibration, as were any readings taken to confirm hypoglycaemia when the sensor alarmed that BSL was “low”. The sensor was removed once baby had been weaned successfully off intravenous dextrose fluids and was normally orally feeding, and data was then downloaded using CareLink® software (Medtronic, Northridge, California).
Graph 1: Driver diagram 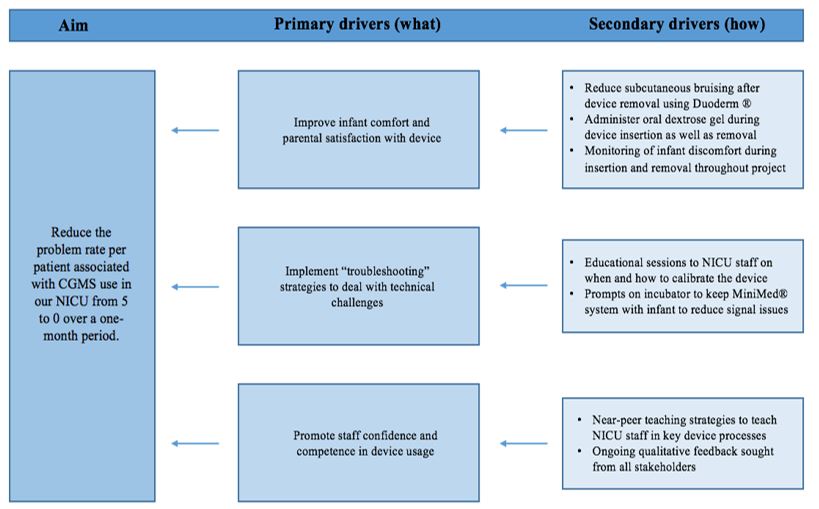
Results
PDSA Cycle One – Would a looser dressing reduce bruising? (Patient 1)
The device was inserted by the primary researcher. In an attempt to reduce subcutaneous bruising noted on the thigh of the pilot infant, the adhesive dressing covering the sensor was loosely applied and not pulled taut over the infant’s skin. While this approach resulted in no bruising, during routine nursing cares this was not secure enough to keep the device in situ and the sensor dislodged.
PDSA Cycle Two – Would an additional protective dressing reduce bruising? (Patient 1)
A layer of Duoderm® dressing was applied to the infant’s thigh underneath the sensor. The device was then re-inserted. Adhesive dressings were then securely applied. No bruising was noted on device removal. We noted that despite using a standard medical adhesive remover, infant discomfort was noted during the removal process (NIPS >3).
Table 1: Characteristics of CGMS monitoring in study infants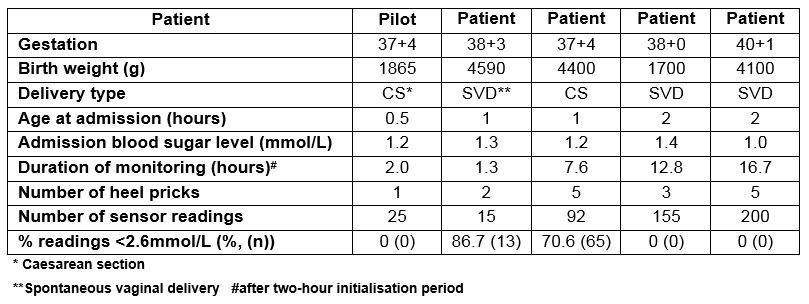
PDSA Cycle Three – Would oral dextrose gel reduce discomfort during device removal? (Patient 2)
As well as a standard medical adhesive remover, oral dextrose gel (0.5ml/kg) was administered to infants prior to both device insertion and removal. Patient 3 scored NIPS 2 on device removal, indicating minimal discomfort. However, we observed that junior doctors and nursing staff caring for the infant were not confident in independently navigating the device without the supervision of study researchers trained in device usage. Discussions with stakeholders revealed a particular confusion about when and how to calibrate the device, with point-of-care glucose readings being taken pre-feed but entered into the device midway or after a feed. This resulted in two calibration errors in a row, necessitating sensor removal.
Table 2: Problems/errors identified during each cycle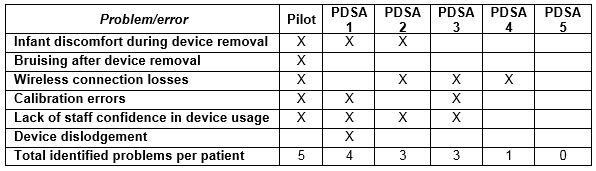
PDSA Cycle Four – Would a simple near-peer teaching method improve NICU staff confidence in CGMS usage? (Patient 3)
The primary researcher used Peyton’s Four-Step Approach to one-on-one successfully teach two junior doctors and two nursing staff how to insert, remove, and correctly calibrate the CGMS device. The sensor was in situ in this patient for several hours. Recurrent wireless signal loss from the sensor and the Minimed® system was noted when the infant was taken out of the incubator for cuddles or cares.
PDSA Cycle Five – Would visual aids prompting parents and staff to keep the CGMS close to the infant result in less signal loss? (Patient 4)
A large notice on the incubator walls with an image of the CGMS device reminded parents and NICU staff to remove the MiniMed® from the incubator along with baby and place it within a metre of the infant. No signal loss was noted during this cycle. Qualitative feedback revealed that the near-peer teaching methods were continuing to promote staff confidence in CGMS usage and thus sustainability of the change intervention.
Graph 2: Project runchart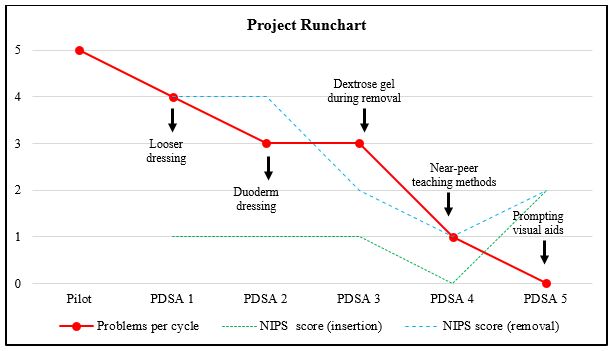
Discussion
This project used basic quality improvement methodologies to implement a change intervention in a structured manner and elucidated aspects of its use that need to be adapted for its successful incorporation into real-life clinical practice. A key factor in this project’s success was that it focused on improving the acceptability and practicality of CGMS use for parents and NICU staff in a busy NICU.
No problems were identified with CGMS in the last PDSA cycle. However, we anticipate that new problems might be inductively identified during future cycles. Additionally, the problems identified within this study context may differ from those identified within another, calling for further quality improvement studies in this area. Furthermore, this study focused on overcoming the initial barriers to CGMS implementation within a unit and not on its use as a clinical management tool. Future quality improvement projects incorporating CGMS into hypoglycaemia management protocols might investigate its potential to improve key clinical outcome measures such as duration of hypoglycaemia or duration of NICU stay.
Conflicts of Interest Statement
The authors declare no conflicts of interest.
Corresponding Author
Dr. Sinead McGlacken-Byrne
Neonatal Intensive Care Unit,
National Maternity Hospital,
Holles St,
Dublin
Email: [email protected]
References
1. Harris DL, Battin MR, Weston PJ. Glucose Monitoring in Newborn Babies at Risk of Hypoglycemia. The Journal of Pediatrics. 2010;157
2. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, VanWeissenbruch M, Midgley P, Cornette L, Ossuetta I, Palmer CR, Iglesias I, De Jong M, Gill B, De Zegher D, Dunger DB. Validation of the continuous glucose monitoring sensor in preterm infants. Archives of Disease in Childhood Fetal and Neonatal edition. 2013;98(2):F136-40.
3. Beardsall K, Ogilvy-Stuart A, Ahluwalia J, Thompson M, Dunger D. The continuous glucose monitoring sensor in neonatal intensive care. Archives of Disease in Childhood Fetal and Neonatal edition. 2005;90(4):F307-F10.
P897
