Factors that Influence Uptake of Vaccination in Pregnancy
Hallissey, R, O’Connell, A, Warren, M
South East General Practice Training Scheme Graduates, 2016
Abstract
Influenza and Pertussis are vaccine preventable diseases carrying significant morbidity and mortality in pregnant women and infants. The uptake of these vaccinations in pregnancy is suboptimal. Our study aimed to identify factors influencing our pregnant patient’s knowledge, beliefs and perceptions. A self-administered, specifically devised, 34-point questionnaire was taken by antenatal patients in a primary care setting from November 2015 to March 2016. A majority believe both diseases are serious during pregnancy or infancy but many are unsure on vaccination safety. A majority of respondents at 80/88 (91%) consider their GP’s advice reliable on this matter. Our key message is that health care provider recommendation is the most powerful tool to improve vaccination uptake. Our research clearly identifies GP’s as the most trusted source of information, making it crucial to deliver accurate information to this high-risk group.
Introduction
Vaccinations against influenza and pertussis are safe, recommended in pregnancy in Ireland and are administered in primary care. Pertussis causes >300,000/year deaths worldwide. In infants less than six months it can cause apnoea, pneumonia and seizures. Evidence suggests that most infants are infected by their mothers1,2. An increase in pertussis worldwide was seen in 2011/2012 with the incidence in Ireland increasing from 114 in 2010 to 458 cases in 20123. There were three neonatal deaths here, all unvaccinated, all younger than 2 months old. Influenza increases the risk of cardiorespiratory complications for pregnant women and heightens risks of premature labour, low birth weight and intrauterine death. It is estimated that immunisation could prevent one to two hospitalisations per 1,000 pregnant women4. Infants under 6 months have the highest rate of death from influenza. In the US, influenza vaccination has been administered to pregnant women since 1957 and is recommended by the Centre for Disease Control (CDC) and American College of Obstetricians and Gynaecologists (ACOG)5.
Both vaccines are inactivated and evidence supports their safe use in pregnancy. No studies have demonstrated an increased risk of either maternal complications or adverse foetal outcomes from influenza vaccine6-8 or tetanus, low dose diphtheria and acellular pertussis (Tdap) vaccine9-13. In Ireland, since August 2012, the National Immunisation Advisory Committee (NIAC) has recommended that pregnant women should be offered Tdap in each pregnancy14. This correlates with similar recommendations in the UK, US, Australia and New Zealand. Influenza vaccination is recommended at any gestation during the flu season. Historically vaccination uptake in pregnancy has been relatively low. Latest UK figures show uptake of influenza and pertussis vaccination in pregnancy are 42.3% and 62.3% respectively15,16.
In order to improve uptake it is important to understand the factors that influence a woman to receive vaccinations during pregnancy. A lreview by Wilson et al in 2015 identified the main barriers related to perceptions of vaccine safety, low knowledge, beliefs that vaccination was not needed or effective, no recommendation from health care workers, access issues, cost and conflicting advice17. Other research reveals that women are more concerned about the potential risks to their infants’ health before their own. They viewed pertussis as more threatening to their infant and so were more likely to vaccinate against it than influenza18. Women who received a recommendation were 7 times more likely to vaccinate than those who had not19.
An Australian based study of 462 participants found that women who mainly attended their general practitioner had a 2.3 times greater chance of receiving the influenza vaccine compared to their counterparts attending the public hospital20. There is very little evidence on vaccination hesitancy in pregnancy in Ireland. We concentrated this research on our practices in the South East, with the aim of understanding the influences and barriers surrounding vaccination uptake amongst our pregnant patients.
Methods
Following literature review, we undertook quantitative research using a self-administered, 34-point questionnaire, given to all pregnant women attending our practices between November 2015 and March 2016. The questionnaire was specifically devised for this study and received ethical approval from the Irish College of General Practitioners Research Ethics Committee. Pregnant patients, 18 years and over, were initially identified from practice software using antenatal protocol and age as search criteria. There are approximately 170 pregnant patients attending the Keogh practice, Waterford city and approximately 30 in Ballyhale, Co Kilkenny, all of whom met our inclusion criteria. Exclusion criteria were age less than 18 years, intellectual disability, those with an acquired brain injury, with life limiting conditions or patients in an emergency setting.
We asked specific questions to assess vaccination history in pregnancy, perceptions regarding vaccine safety and efficacy, knowledge, attitudes and beliefs surrounding vaccines, disease severity and potential for attitudinal change based on physician recommendation. Each researcher presented the proposed study at a practice meeting and practical amendments were made based on participating staff feedback. Ethical approval was obtained at this point. Staff were kept updated about study progress when research commenced and concluded. A4 size posters were placed in practice waiting rooms alerting patients that this research was being undertaken. Patient information leaflets about both vaccines were placed in all consultation rooms and public areas. The questionnaire was given to patients when checking in for their routine antenatal appointment. A consent form outlining confidentiality, anonymity and permission to decline was presented along with a research study information leaflet, allowing the patient to make a fully informed choice before participation.
On return of these documents to practice staff and irrespective of their choice to participate, all patients were provided with further written information. This detailed the availability to them of both vaccines as part of standard, nationally recommended antenatal care. A study investigator or the patient’s own GP provided further information at the antenatal appointment if the patient so wished. Randomisation was not required as all attending for routine antenatal care were invited to participate. Data was anonymized and questionnaires were then coded numerically for analysis. We used Microsoft Excel for calculation and interpretation of our results.
Results
All our study participants were pregnant women, of mean age 31 years (range 18-42 years), with representation from all trimesters. Two hundred patients were invited to participate of whom 88 completed surveys (44%). The timeframe for participation was December 2015 to March 2016, overlapping with flu season. The majority of respondents, 65/88 (74%) were private patients and 67/88 (76%) spoke English as a first language. The majority, 68/88 (77%) had third level education and 65/88 (74%) were engaged in full or part time work outside the home.
Antenatal vaccine awareness and uptake
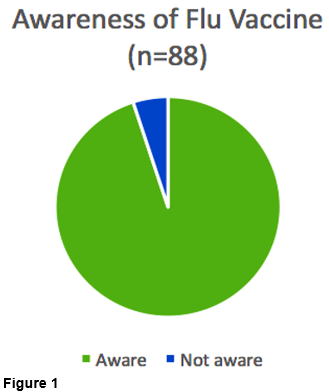
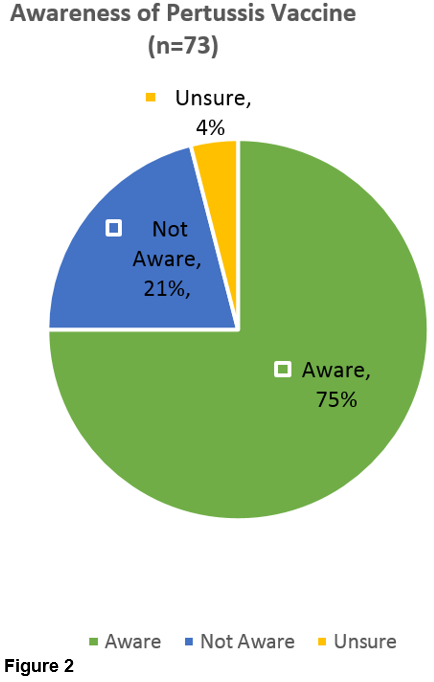
We found that 84/88 (95%) were aware of flu vaccine (Figure 1). Fewer were aware of pertussis vaccine at 55/73 (75%) (Figure 2).
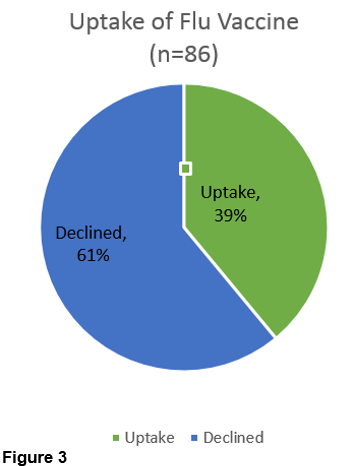
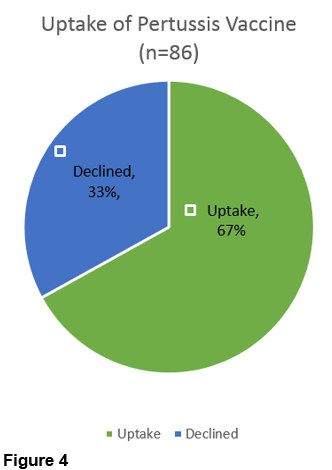
However uptake levels at 58/86 (67%) was higher for the pertussis vaccination (Figure 4) than for flu vaccine at 34/86 (40%) (Figure 3).
Perception of risk
Of total 88 participants, 58/88 (66%) recognise that contracting flu during pregnancy could be serious for themselves. A larger number, 70/88 (80%) felt the illness could seriously impact the health of new-borns. Forty-eight respondents of 88 (55%) believe flu vaccine is safe for them in pregnancy but 35/88 (40%) were unsure if it posed danger to their unborn baby. Most respondents, 57/88 (65%) think contracting whooping cough while pregnant could be serious for themselves with 69/88 (78%) feeling that pertussis is dangerous for new-borns. Forty four participants of 88 (50%) were unsure if pertussis vaccine could harm them or their baby.
Information source reliability
The overwhelming majority, 80/88 (91%), find their GP to be a reliable source of information on vaccination in pregnancy, closely followed by their Obstetrician or hospital clinic at 71/88 (81%).
Discussion
Immunisation is one of the most effective interventions in modern medicine, conferring a reduction in global infectious disease burden and even eliminating some conditions previously responsible for significant suffering and death. Pregnant women and young infants represent two uniquely susceptible groups for increased morbidity and mortality from influenza and pertussis. Thus, immunisation against both diseases is recommended in Ireland and internationally. Vaccination of pregnant patients offers a single intervention that will protect two individuals: direct immunity is conferred on the susceptible antenatal patient and maternally driven antibodies offer vital neonatal protection via placental transfer. Evidence indicates that women are more concerned about potential risks to their infant’s health than their own and it has been shown that women were more likely to vaccinate against pertussis than flu due to a perception of it being more serious for new-borns17. This may account for our finding that, even though fewer participants were aware of the pertussis vaccine it had a relatively higher uptake than flu vaccine.
There is an increasing body of evidence to support the safety and health benefits of vaccinations in pregnancy5,12,22. Indeed, it is likely that with ongoing research the number of vaccines recommended during pregnancy will increase22. Our study demonstrates a lot of uncertainty amongst patients surrounding vaccine safety as well as a lack of knowledge about the risks posed to themselves and their babies from influenza and pertussis. Positive health messages emphasizing immunisation benefits for their infants would encourage antenatal patients to vaccinate17. Post education uptake has been demonstrated to increase in the literature16. The key message of our study and literature review is the following: health care provider recommendation is the most powerful tool to improve vaccination uptake. Our research clearly identifies GPs as the most trusted source of information to pregnant women, largely correlating with literature that doctors and health care providers as dependable and trustworthy in this context18-22,24. Therefore, it is crucial that GPs deliver accurate information to this high-risk group. To give the right advice we have a responsibility to address the misconceptions and knowledge gaps studies have proven we hold20,21,23,25. This will then allow us to address the numerous modifiable patient barriers that exist16,20-24. This was a prospective cohort study of only 88 patients in one geographical area of Ireland. However the sample represents the diverse population of women attending urban and rural GP practices from a variety of cultural and ethnic backgrounds.
We relied on self-reported vaccination uptake status. An element of selection bias may exist where patients interested in study participation are more motivated with regard to health seeking behaviours. Certain survey questions were left unanswered. We postulate that private patients may be over-represented in our cohort because they were easier to identify by reception staff during the study recruitment phase; private pateints must clearly indicate that they are attending for an antenatal visit, which is funded by the Mother and Infant scheme. At time of authorship pertussis vaccination is recommended from week 27-36 of pregnancy so the survey may have been taken too early for some. Circulating our survey during the time of seasonal flu vaccination may have introduced positive bias with regard to awareness.
We conclude that if targeted education on immunisation during pregnancy has such a positive impact on maternal vaccination coverage, then an onus is on us as health care providers to facilitate this. Our own beliefs, attitudes and knowledge gaps must be acknowledged in the first instance. We suggest further research into opinions of all Irish antenatal care providers would be valuable in light of studies showing vastly inceased uptake amongst patients whose health care provider advocated for vaccination.20,21
The majority of pregnant women in Ireland encounter their GP, Obstetric doctors and midwives during routine antenatal care. Besides the establishment of a trusting relationship between patient and provider, the frequency of the scheduled appointments affords multiple opportunities to address vaccination. We recommend that hospital based Obstetrics teams prioritise internal educational sessions on the importance of vaccination in pregnancy carried out in Primary care. Written information leaflets should be routinely provided to patients in antenatal packs. Local CME groups represent an excellent platform for dissemination of accurate information to GPs.
Correspondence
Dr Ann O’Connell, 19 Summerville Avenue, Waterford
Email: [email protected]
Conflict of Interest
None to declare
References
1) Bisgard K, Pascual F, Ehresmann K, Miller C, Cianfrini C, Lett S. Infant pertussis: who was the source?. The Pediatric Infectious Disease Journal. (2004, Nov) ; 23(11): 985-989.
2) Wendelboe A, Njamkepo E, Bourillon A, Floret D, Gaudelus J, Van Rie A. Transmission of Bordetella pertussis to Young Infants. Pediatric Infectious Disease Journal. (2007, Apr); 26(4): 293.
3) HPSC annual report 2012. Chapter 1, vaccine preventable diseases, Pertussis 1.6, 26.
4) Neuzil K, Reed G, Mitchel E, Simonsen L, Griffin M. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. American Journal Of Epidemiology. (1998, Dec 1), 148(11): 1094-1102.
5) Shavell V, Moniz M, Gonik B, Beigi R. Influenza immunization in pregnancy: overcoming patient and health care provider barriers. American Journal Of Obstetrics And Gynecology. (2012, Sep); 207(3 Suppl): S67-S74.
6) Nordin J, Kharbanda E, Benitez G, Nichol K, Lipkind H, Olsen A. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstetrics And Gynecology. (2013, Mar),121(3): 519-525.
7) Kharbanda E, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin J. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstetrics And Gynecology (2013, Sep), 122(3): 659-667.
8) Tamma PD, Ault KA, del Rio C. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2009; 201:547.
9) Munoz F, Bond N, Maccato M, Pinell P, Hammill H, Baker C. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. Jama. (2014, May 7); 311(17): 1760-1769.
10) Zheteyeva Y, Moro P, Tepper N, Rasmussen S, Barash F, Broder K. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. American Journal Of Obstetrics And Gynecology. (2012, July); 207(1): 59.e1-7.
11) Shakib J, Korgenski K, Sheng X, Varner M, Pavia A, Byington C. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. The Journal Of Pediatrics. (2013, Nov), 163(5): 1422-6.e1-4.
12) Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ (Clinical Research Ed.) [serial on the Internet]. (2014, July 11), [cited November 16, 2016]; 349g4219.
13) Kharbanda E, Vazquez-Benitez G, Lipkind H, Klein N, Cheetham T, Nordin. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. Jama (2014, Nov 12), 312(18): 1897-1904.
14) Public Health England. Seasonal influenza vaccine uptake amongst GP Patients in England. Provisional monthly data for1 September 2015 to 31 January 2016. Available from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/503124/January_2016_Seasonal_flu_GP_patients_01Sept_31Jan.pdf
15) Public health England. Pertussis vaccination programme for pregnant women: vaccine coverage estimates in England, September to December 2014. Available from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/408500/hpr0715_prtsss-vc.pdf
16) Wilson R, Paterson P, Jarrett C, Larson H. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine. (2015, Nov 25), 33(47): 6420-6429.
17) Wiley K, Cooper S, Wood N, Leask J. Understanding Pregnant Women’s Attitudes and Behavior Toward Influenza and Pertussis Vaccination. Qualitative Health Research. (2015, Mar) ; 25(3): 360.
18) Wiley K, Massey P, Cooper S, Wood N, Quinn H, Leask J. Pregnant women's intention to take up a post-partum pertussis vaccine, and their willingness to take up the vaccine while pregnant: a cross sectional survey. Vaccine. (2013, Aug 20); 31(37): 3972-3978.
19) Maher L, Hope K, Torvaldsen S, Lawrence G, Dawson A, Conaty S. Influenza vaccination during pregnancy: coverage rates and influencing factors in two urban districts in Sydney. Vaccine. (2013, Nov 12), 31(47): 5557-5564.
20) Ozer A, Arikan D, Kirecci E, Ekerbicer H. Status of pandemic influenza vaccination and factors affecting it in pregnant women in Kahramanmaras, an eastern Mediterranean city of Turkey. Plos One. (2010, Dec 1); 5(12): e14177.
21) Moniz M, Beigi R. Maternal immunization. Clinical experiences, challenges, and opportunities in vaccine acceptance. Human Vaccines & Immunotherapeutics. (2014); 10(9): 2562-2570.
22) Regan A, Moore H, de Klerk N, Omer S, Shellam G, Effler P. Seasonal Trivalent Influenza Vaccination During Pregnancy and the Incidence of Stillbirth: Population-Based Retrospective Cohort Study. Clinical Infectious Diseases: An Official Publication Of The Infectious Diseases Society Of America. (2016, May 15), 62(10): 1221-1227.
23) Chamberlain A, Seib K, Ault K, Orenstein W, Frew P, Omer S. Factors Associated with Intention to Receive Influenza and Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccines during Pregnancy: A Focus on Vaccine Hesitancy and Perceptions of Disease Severity and Vaccine Safety. Plos Currents. (2015, Feb 25), 7
24) Bödeker B, Walter D, Reiter S, Wichmann O. Cross-sectional study on factors associated with influenza vaccine uptake and pertussis vaccination status among pregnant women in Germany. Vaccine. (2014, July 16, 32(33): 4131-4139.
25) Broughton D, Beigi R, Switzer G, Raker C, Anderson B. Obstetric health care workers' attitudes and beliefs regarding influenza vaccination in pregnancy. Obstetrics and Gynecology. (2009, Nov), 114(5): 981-987.
(P713)
