High Rates of Diabetic Ketoacidosis in Patients with New and Known Type 1 Diabetes over a Six-Year Period
B.P. Finn, J. Trayer, C. Cronin, S.M. O’Connell
Department of Paediatrics and Child Health, Cork University Hospital
Abstract
Aims
To analyse all paediatric patients who presented with diabetic ketoacidosis (DKA) from 2012 to 2017.
Methods
A retrospective observational study was carried out analysing all cases of diabetic ketoacidosis admitted to a regional centre from 2012-2017.
Results
We identified 133 cases of DKA, 81 (61%) were newly diagnosed patients and 52 (39%) were patients with known T1DM. There were 215 new diagnoses of T1DM during the study period giving a DKA rate at diagnosis of 38%. Among the 52 cases with established T1DM, 13 cases (25%) presented in severe DKA and 37 cases (71%) occurred in adolescents aged over 12 years. Precipitating factors included chronic suboptimal control and psychosocial factors (28/52), acute illness (16/52), and pump technical failure (5/52). There were two cases treated for suspected cerebral oedema and one case each of subarachnoid haemorrhage and cardiac arrhythmia.
Conclusion
The current proportion of new T1DM presenting in DKA is higher than international data. The high frequency of DKA in known T1DM indicates a need for particular focus on adolescents.
Introduction
Ireland is a high incidence country for type 1 diabetes (T1DM) with 28.8 newly diagnosed cases/100,000/year1. Patients with new onset T1DM frequently (15-70%) present with diabetic ketoacidosis (DKA)2,3,4,5. Lansdown et al found that 25% of children with new onset T1DM under 19 years of age presented in DKA in Wales between 1991 and 20096. The rates of DKA in known T1DM on an international stage remains significant- Austria and Germany (5%), Wales and England (6.4%) and the United States (7.1%)7. The aim of our study was to analyse all admissions with diabetic ketoacidosis to the regional paediatric diabetes centre at Cork University Hospital, over the past six years. Our objectives include assessing the proportion of patients with new and known T1DM, treatment modalities (e.g. pump/multiple daily injections), patient demographics, severity, length of stay and outcomes.
Methods
This was a retrospective chart review of all presentations with DKA to our centre from 1st January 2012 to 31st December 2017. Patients were identified from prospectively maintained records kept in the Paediatric Diabetes service at Cork University Hospital (CUH) from 2015-2017. For 2012-2014, the medical Hospital In-Patient Enquiry (HIPE) records provided a list of all admissions with T1DM. All patients aged 0 to 18 years of age who were admitted under the care of the paediatric department were included in the study. We defined Diabetic Ketoacidosis as per the British Society for Paediatric Endocrinology and Diabetes (BSPED) 2015 criteria of pH <7.3 or bicarbonate <18mmol/L with evidence of ketonaemia (>3mmol/L) or hyperglycaemia (blood glucose >11mol/L)8. Severity of DKA was further classified as Mild: pH<7.3, bicarbonate <15, Moderate: pH<7.2, bicarbonate<10 or Severe: Ph<7.1, bicarbonate <5.
The preceding factors or precipitant for DKA in known patients with T1DM were defined for the purposes of this study as follows: “Illness” - A preceding illness e.g. gastroenteritis or a respiratory tract infection, which required implementation of sick day rules but where the sick day rules were not successful. Gastroenteritis was determined on the basis of additional symptoms including diarrhoea and sick contacts. “Poor compliance” is where the patient stopped taking their insulin, omitted an insulin dose or failed to adequately dose for hyperglycaemia leading to DKA. “Suboptimal Control” describes an elevated HbA1C >7.5% noted prior to presentation with DKA9. “Psychosocial issues” refers to patients with a significant history of deliberate self harm, family conflict and social issues at home or in school or alcohol use which were impacting on their management of diabetes and leading to presentation in DKA. “Pump failure” refers to a technical fault with an insulin pump leading to failure to deliver insulin including problems with insulin delivery due to a dislodged or blocked infusion set.
Cork University Hospital uses the 2009 BSPED DKA guidelines for all patients who present in DKA with the standard dose of 0.1units/kg of insulin for all patients.
Hypokalaemia was defined as K+ <3.5mmol/L.
The results were collated on a Microsoft excel spreadsheet and subsequently analysed using Stata 151C.
Continuous data was summarised as mean (standard deviation) and categorical data as frequency (percentage). Tests for trends over time were assessed by including calendar year as a covariate in an appropriate regression model; logistic regression was used for binary data, ordered logistic regression for ordinal data and linear regression for continuous. When assessing trends in treatment characteristics and complication rates, models were also adjusted for severity of DKA. Other comparisons were carried out using the Chi-square test.
Results
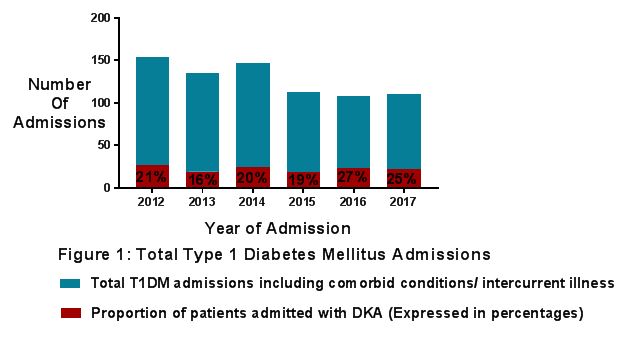
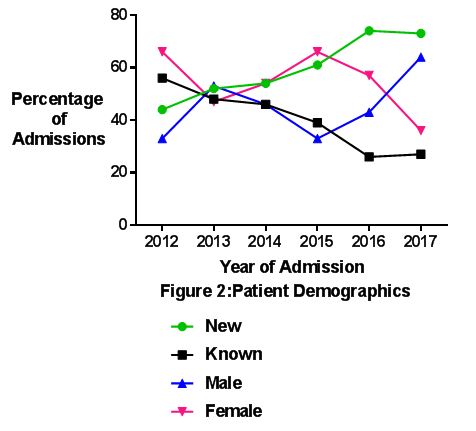
The total number of patients with T1DM admitted over the six-year period was 634 cases of which 133 cases (21%) were in DKA. Figure 1 and Table 1 show a breakdown of those presenting with or without DKA as a percentage of the total for each year. There were no significant trends over time in the proportion of patients who were in DKA at admission overall (p=0.176) or among new (p=0.598) and existing (P=0.936) T1DM patients. Among patients with DKA, those already known to have T1DM made up 52 out of 133 cases (39%) (Figure 2). The proportion of cases in which T1DM was known decreased significantly from 56% (15 of 27 cases) in 2012 to 27% (6 of 22 cases) in 2017 (p=0.011). The number of new onset T1DM patients who presented in DKA was 38% over the 6 year period (p=0.598) of which 53% presented in moderate or severe DKA (Table 1). In short 20% of new onset T1DM patients presented in moderate or severe DKA.
There was a slight preponderance towards the female gender (n=71, 55% of admissions) with no significant difference in the proportion of females over time (p=0.595) The mean age at presentation of DKA did not vary over time (p=0.313) with patients presenting at a mean age of 10.5 (SD 4.6) years and ranging in age from 0.75 to 17.58 years. Patients with existing diabetes were significantly older (mean 13.5, SD 3.2 years) than those with new onset diabetes (mean 8.5, SD 4.4 years) (p<0.001). Four adolescent females accounted for 17 admissions for DKA amongst the known patients. Taking into account these recurrent presentations, by treating these 4 patients as one case each (n=4) rather than 17 cases of DKA, then DKA in known T1DM only makes up 32.5% of DKA presentations (39/120).
Overall, the severity of DKA was mild in 61 (46%), moderate in 34 (25.5%) and severe in 38 (28.5%) cases (Table 1) and did not vary over time (p=0.222) (Table 1). Of the 52 with known diabetes, 13 (25%) had severe DKA, 16 (31%) had moderate DKA but the differences in severity were not statistically significant (p=0.524).
Table 1: Trends in admissions and severity of DKA in patients with T1DM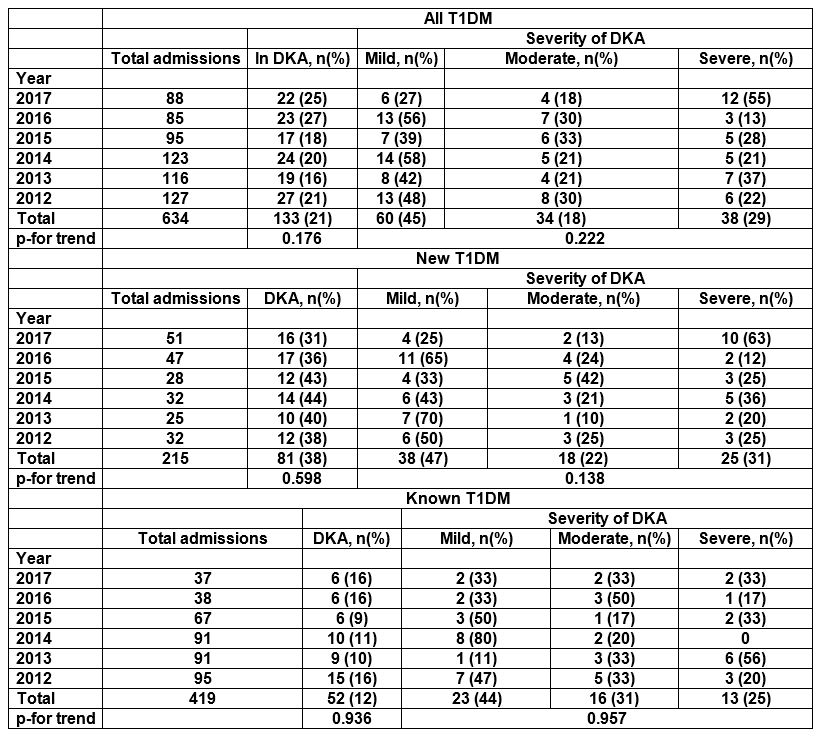
 The mean time to acidosis correction was 18.3 (SD 9.6) hours with a mean of 30.7 (SD 13.4) hours on the DKA protocol and 5.7 (SD 2.4, p=0.168) days spent in hospital including acute care, re-education and support (P-values were adjusted for severity of DKA). The average length of stay for a patient with known T1DM is 3.8 days, shorter than the 6.5 days for new onset T1DM. Ten (8%) of patients required an ICU admission (p=0.104), for 1-2 days at most. One hundred and twenty three (92%) of patients who did not receive ICU care required one to one nursing care in the form of a high dependency care delivery on the general paediatric ward. Hypokalaemia as a complication of DKA occurred in 16% of DKA cases (n=21, p=0.884). Between 2012 and 2017 there were no deaths, no occurrences of seizures or line thrombosis. There were two cases treated for suspected cerebral oedema (CO) and one case each of subarachnoid haemorrhage and cardiac arrhythmia; all had good outcomes.
The mean time to acidosis correction was 18.3 (SD 9.6) hours with a mean of 30.7 (SD 13.4) hours on the DKA protocol and 5.7 (SD 2.4, p=0.168) days spent in hospital including acute care, re-education and support (P-values were adjusted for severity of DKA). The average length of stay for a patient with known T1DM is 3.8 days, shorter than the 6.5 days for new onset T1DM. Ten (8%) of patients required an ICU admission (p=0.104), for 1-2 days at most. One hundred and twenty three (92%) of patients who did not receive ICU care required one to one nursing care in the form of a high dependency care delivery on the general paediatric ward. Hypokalaemia as a complication of DKA occurred in 16% of DKA cases (n=21, p=0.884). Between 2012 and 2017 there were no deaths, no occurrences of seizures or line thrombosis. There were two cases treated for suspected cerebral oedema (CO) and one case each of subarachnoid haemorrhage and cardiac arrhythmia; all had good outcomes.
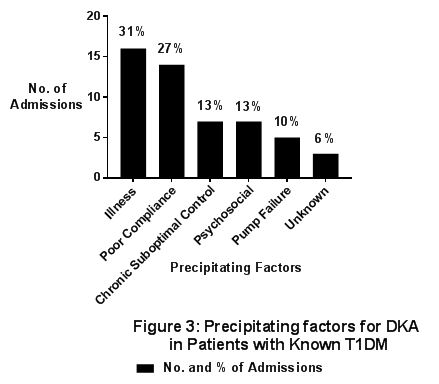
The proportion of patients who presented directly to the ED (rather than to a GP) increased from 41% (11 of 27 cases) in 2012 to 59% (13 of 22 cases) in 2017, though this was not statistically significant (p=0.188). The majority (77%) of children with new T1DM presented to a GP, with GPs missing the diagnosis in 10% of all children with new T1DM. The rates of presentation to a GP versus direct to A&E in those with new T1DM did not vary over time (p=0.264). Parents recognised signs and symptoms in the vast majority of cases of DKA in the children with known T1DM, with 85% (44 of 52) taking their child directly to the ED while 15% (8 of 52) attended their local GP first due to uncertainty regarding the diagnosis. There is 100% parental recognition of DKA in patients with known T1DM over the past 3 years, a significant increase over time rising from 60% in 2012 (p=0.003). General practitioners only missed 3 cases (8%) of severe DKA but still appropriately referred the child to ED. The reasons for this include mistaking DKA for an infective process e.g. UTI, gastroenteritis, urinary tract infection or oral thrush, and in one case the child's vomiting was also put down to a sequela of a head injury. The precipitating factors for DKA are shown in figure 3. Three cases could not be categorised as no precipitating cause was identified.
Financial Costs
Seventeen nights in total were spent by patients in the ICU with a cost per night of €1,500. Therefore, over the six-year period the cost of treating patients with DKA admitted to the ICU was €25,500.
Treatment of patients admitted with DKA who spent a total of 769 nights (€646 per night) as inpatients on the paediatric ward excluding the nights spent in ICU cost a total of €496,774 over the six-year period.
Of this, patient with known T1DM spent 197 nights as inpatients with no nights spent in ICU, resulting in a cost of €127,262 over the past 6 years.
The total final financial cost of treatment of DKA over the six-year period was €522,274.
Discussion
Our study demonstrates a similarly high rate of new patients presenting in DKA (mean 38%) over the past six years with 20% of new onset T1DM presenting in moderate or severe DKA. This is an improvement in the severity of DKA when compared to the national figures from 1997-1998 of 25% of new patients presenting in moderate or severe DKA (pH<7.2, bicarbonate <15 mmol/L, blood glucose >15mmol/L) with a further 6% treated as ''suspected'' (mild DKA defined as pH 7.2-7.3)10. A similar single national tertiary centre in Ireland found that 28.7% of children with new onset T1DM presented in DKA from 2008-2012, with 15.5% presenting in moderate or severe DKA 11. This study demonstrates a persistently high rate of DKA at diagnosis in Ireland as does our own study. It also supports that the severity of DKA at diagnosis in Ireland has improved over the past 20 years. The majority of patients present in mild DKA, however, severe DKA is more likely in new onset T1DM.
The reasons for patients with known diabetes becoming acidotic are primarily due to intercurrent illness, most commonly gastroenteritis and upper respiratory tract infections or due to poor compliance. Four patients presented recurrently accounting for 17 admissions, where identified risk factors included chronic suboptimal glycaemic control and psychosocial issues. All 4 were female adolescents which is a known risk factor for recurrent DKA12-15.
Other risk factors for recurrent DKA are poor glycaemic control, family conflict and psychiatric disorders as reflected in our data. Two adolescent females (as reported above) each presented 5 and 7 times respectively in DKA. These recurrent episodes were often due to family conflict and attempts at deliberate self-harm by omitting insulin deliberately in successful attempts at inducing acidosis. Our experience supports the need for extra resources for adolescent diabetes clinics where there can be a focus on raising awareness of the dangers of and precipitating factors for DKA.
The increased rates of direct presentation to ED in known T1DM suggests increased parental recognition over the past few years of DKA. Only one patient with known T1DM presented to ED where the signs of DKA had not been appreciated, which suggests good knowledge and appreciation of “sick day rules”.
Patients presenting with DKA present in two distinct groups – those with new onset diabetes where focus on early recognition by parents and GPs should be prioritised, and those with known diabetes who need support and education to avoid this potential complication of technology failure, psychosocial and compliance issues. Ideally patients with known T1DM should not present in DKA, by using measures to recognise symptoms and signs of illness, pump failure and increased insulin requirements such as hyperglycaemia and ketosis before ketoacidosis ensues. The recognition of these factors by families, patients and carers is improved by regular review of “sick day rules” and encouraging close telephone contact with the paediatric diabetes team and medical staff if a child becomes unwell. Admission rates with DKA should be considered a key performance indicator (KPI) of diabetes management and rates of presentation should be compared nationally, and with international literature. In patients who present with recurrent DKA, the multi-disciplinary team (MDT) has a huge role in management of these individual high-risk patients and close work with the families and patients themselves, to offer alternative means of insulin administration and assistance from clinical psychology, and child and adolescent mental health services where indicated. Re-audit and quality improvement remain a huge component of this KPI. The aim of monitoring our DKA rates is not just to care for children in the acute setting but to safeguard our patients’ future neurocognitive development and mental health. Good glycaemic control and prevention of acute complications such as DKA can prevent long term effects such as lower school performance and executive function16-18.
Conflicts of Interest Statement
None
Acknowledgements
We would like to especially thank the following members of the Endocrinology Department in Cork University Hospital: Dr Stephen O'Riordan, Paediatric Endocrinologist, Ms Shirley Beattie Paediatric Diabetes Dietitian, Ms Anne Bradfield and Ms Laura Crowley Paediatric Diabetes Nurse Specialists and Prof Jonathan Hourihane for his advice and support.
Contributorship Statement
Bryan Finn: Literature search, data collection, analysis and interpretation, writing and figures
James Trayer: Data collection, analysis and interpretation
Conor Cronin: Data collection
Susan M O'Connell: Study design, literature search, data collection, data interpretation and writing.
Corresponding Author
Bryan Padraig Finn
Department of Paediatrics and Child Health,
Cork University Hospital
Email: [email protected], [email protected]
References
1. Roche E, McKenna A, Ryder K, Brennan A, O’Regan M, Hoey H. Is the incidence of type 1 diabetes in children and adolescents stabilising? The first 6 years of a National Register. European Journal of Pediatrics. 2016;175(12):1913-1919.
2. Craig M, Jefferies C, Dabelea D, Balde N, Seth A, Donaghue K. Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric Diabetes. 2014;15(S20):4-17.
3. Usher-Smith J, Thompson M, Sharp S, Walter F. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343(jul07 1):d4092-d4092.
4. Rewers A, Klingensmith G, Davis C, Petitti D, Pihoker C, Rodriguez B, Schwartz ID. Imperatore G, Williams D, Donal LM, Dabelea D. Presence of Diabetic Ketoacidosis at Diagnosis of Diabetes Mellitus in Youth: The Search for Diabetes in Youth Study. PEDIATRICS. 2008;121(5):e1258-e1266
5. Usher-Smith J, Thompson M, Ercole A, Walter F. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55(11):2878-2894.
6. Lansdown AJ, Barton J, Warner J, Williams D, Gregory JW, Harvey JN and Lowes L. Prevalence of ketoacidosis at diagnosis of childhood onset Type 1 diabetes in Wales from 1991 to 2009 and effect of a publicity campaign. Diabetic Medicine. 2012;29(12):1506-1509
7. Maahs DM, Hermann JM, Holam N, Foster NC, Kapellen TM, Allgrove J, Schatz DA, Hofer SE, Camplbell F, Steigleder-Schweiger C, Beck RW, Warner JT, Holl RW. Rates of Diabetic Ketoacidosis: International Comparison With 49,859 Pediatric Patients With Type 1 Diabetes From England, Wales, the U.S., Austria, and Germany. Diabetes Care. 2015;41(4):1-7
8. Edge J. BSPED Recommended Guideline for the Management of Children and Young People under the age of 18 years with Diabetic Ketoacidosis 2015. BSPED Committee. https://www.bsped.org.uk/media/1381/dkaguideline.pdf
9. Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatric Diabetes 2007: 8: 408–418.
10. Roche E, Menon A, Gill D, Hoey H. Clinical presentation of type 1 diabetes. Pediatric Diabetes. 2005;6(2):75-78.
11. McKenna A, Sandys N, Ryder K, Metwally, N, Brennan A, O’Regan M, Hoey MCV, Roche EF
Single Centre Experience - Clinical Presentation and frequency of Paediatric Diabetic Ketoacidosis (DKA) at diagnosis over a 5-year period. IMJ. 2018; 11(3):706-714.
12. O'Grady M, Delaney J, Jones T, Davis EA. Standardised mortality is increased three-fold in a population-based sample of children and adolescents with type 1 diabetes. Pediatric Diabetes. 2012;14(1):13-17.
13. Rewers A. Predictors of Acute Complications in Children with Type 1 Diabetes. JAMA. 2002;287(19):2511.
14. Bui T, Werther G, Cameron F. Trends in diabetic ketoacidosis in childhood and adolescence: a 15-year experience. Pediatric Diabetes. 2002;3(2):82-88.
15. Smith C, Firth D, Bennett S, Howard C, Chisholm P. Ketoacidosis occurring in newly diagnosed and established diabetic children. Acta Paediatrica. 1998;87(5):537-541.
16. Northam E, Rankins D, Lin A, Wellard R, Pell G, Finch S, Werther GA, Cameron FJ.
Central Nervous System Function in Youth with Type 1 Diabetes 12 Years After Disease Onset. Diabetes Care. 2009;32(3):445-450.
17. Hannonen R, Komulainen J, Riikonen R, Ahonen T, Eklund K, Tolvanen A, Keskinen P, Nuuja A. Academic skills in children with early-onset type 1 diabetes: the effects of diabetes-related risk factors. Developmental Medicine & Child Neurology. 2012;54(5):457-463.
18. Cameron F, Scratch S, Nadebaum C, Northam E, Koves I, Jennings J, Finney K, Neil JJ, Wellard RM, Mackay M, Inder TE on behalf of the DKA brain injury study group. Neurological Consequences of Diabetic Ketoacidosis at Initial Presentation of Type 1 Diabetes in a Prospective Cohort Study of Children. Diabetes Care. 2014;37(6):1554-1562.
P898
