Is It Time To Review The Vaccination Strategy To Protect Adults Against Invasive Pneumococcal Disease?
M. Corcoran1, J. Mereckiene2, S. Murchan2, M. McElligott1,3,, D. O’Flanagan2, S. Cotter2, R. Cunney1,2,4, H. Humphreys1,3,5
1. The Irish Pneumococcal Reference Laboratory, Irish Meningitis and Sepsis Reference Laboratory, Temple Street Children’s University Hospital, Temple Street, Dublin 1, Ireland.
2. Health Protection Surveillance Centre, Dublin, Gardiner Street, Dublin 1, Ireland.
3. Department of Clinical Microbiology, the Royal College of Surgeons in Ireland, RCSI Education & Research Centre, Beaumont Hospital, Beaumont, Dublin 9, Ireland.
4. Department of Microbiology, Temple Street Children’s University Hospital, Dublin 1, Ireland.
5. Department of Microbiology, Beaumont Hospital, Beaumont, Dublin 9, Ireland.
Abstract
Pneumococcal conjugate vaccines (PCVs) have reduced the predominant serotypes causing invasive pneumococcal disease (IPD). We assessed the impact of the paediatric 7- and 13-valent pneumococcal conjugate vaccines (PCV7 and PCV13) among older adults. We compared serotype-specific incidence rates from 2007/08 to 2016/17, expressed as incidence rate ratios (IRR).
Introducing PCV7 and PCV13 into the childhood immunisation programme resulted in a decline in these serotypes in adults ≥65 years of age, with PCV7 serotypes decreasing by 85% (IRR=0.11, 95%CI: 0.05-0.22, p<0.0001) and PCV13 serotypes not included in PCV7 (PCV13-7), decreasing by 9% (IRR=0.68, 95%CI: 0.40-1.16, p=0.134). However, there was a significant increase in serotypes only found in the 23-valent polysaccharide vaccine, PPV23-PCV13: IRR=2.57, 95%CI: 1.68-4.03, p<0.0001, and non-vaccine types (NVTs), IRR=3.33, 95%CI: 1.75-6.84, p=0.0001.
The decline of IPD associated with PCV7/13 serotypes and the increase in PPV23-PCV13 serotypes indicates clear serotype replacement. Increasing PPV23 uptake could still reduce the burden of disease for this population.
Introduction
Streptococcus pneumoniae frequently colonises the nasopharynx in healthy people asymptomatically, but can cause a wide spectrum of disease ranging from non-invasive infections (e.g. acute otitis media, sinusitis and pneumonia) to invasive pneumococcal disease (IPD), including bloodstream infections (septicaemia) and meningitis, which are a considerable cause of morbidity and mortality. In the United States, the Centers for Disease Control and Prevention (CDC) estimate the highest incidences of IPD occur in adults ≥65 years of age and children <2 years. Much higher mortality rates are now associated with the older age groups (approx. 18/100000 in those aged ≥65 years) than in the young (approx. 0.4/100,000 in children <2 years)1.
The incidence of IPD in young children has fallen significantly in most countries due to the introduction of pneumococcal conjugate vaccines (PCVs) which provide protection against the predominant serotypes circulating in children2-4. The 7-valent vaccine (PCV7) was introduced in Ireland in September 2008 and the higher 13-valent pneumococcal conjugate vaccine (PCV13) in December 2010. The uptake of the recommended 3 doses at 24 months of age has been consistently >90% in recent years5. The number of PCV7 and PCV13-7 cases (i.e. the six additional serotypes that are in PCV13 only) have fallen by 100% and 81%, respectively, in patients <5 years of age and is similar to what is reported elsewhere with a 3 dose schedule6-9. Overall in the Irish population, the number of confirmed cases of IPD in Ireland has fallen by 10% since vaccination commenced in 2008, with a greater decline in PCV associated serotypes observed in all age categories, which is suggestive of herd immunity as a result of paediatric immunisation.6
However, despite some positive impact for adults, the overall incidence of IPD has remained high in adults in Ireland10. Since the 1980s, it is recommended that all adults aged ≥65 years of age receive PPV23. In addition to this, since 2015 those with risk-associated co-morbidities, irrespective of age, should receive PPV23 and one dose of PCV13 11.
Recent data indicates that 69.4% of adults ≥65 years of age have a medical/GP visit card and with all adults >70 years of age eligible for free GP care since 2015, these patients are entitled to receive the vaccine free of charge12. Despite this, the uptake of PPV23 remains low in Ireland, with just 36% of adults ≥65 years of age vaccinated as of 201313. This is in contrast to other countries such as the United Kingdom where uptake is as high as 70%14. A previous review indicated that Ireland had the 3rd highest number of doses of PPV23 distribution in Europe (1,488/10,000 population)15, however, this may not fully reflect direct uptake. We were unable to attain further information on vaccine uptake or dose distribution in this population from public health records or other sources.
Given changes arising from the introduction of the PCV vaccines in children, we analysed national serotype data for IPD in the adult population to determine if changes in the vaccination strategy need to be considered, especially as admission to hospital with sepsis or pneumonia increases the likelihood of readmission with cardiovascular disease, coronary disease and stroke16.
Methods
Clinicians and laboratories are legally obliged to notify the local public health departments of IPD cases in Ireland since 2004. This includes IPD cases confirmed by culture or by the detection of nucleic acid or antigens from a normally sterile site. Data are collated using the Computerised Infectious Diseases Reporting (CIDR) system, a secure web-based system for collecting and collating data on notifiable infectious diseases in Ireland. The Irish Pneumococcal Reference Laboratory (IPRL) was established in April 2007 in order to assess the serotypes associated with invasive disease prior to the introduction of the conjugate vaccines. Public and private clinical laboratories are requested to refer all culture positive IPD isolates to IPRL for typing. The serotype results are linked with the individual notification events reported each quarter using the CIDR system with the assistance of the Health Protection Surveillance Centre (HPSC) and local public health departments.
Serotyping was performed on available IPD isolates from adults aged ≥65 years of age by serological reactions using serum samples from Staten Serum Institut (SSI Diagnostica, Hillerød, Denmark) and a multiplex-PCR as previously described17. Antimicrobial susceptibility was assessed using the ETEST®method, and results interpreted using European Committee on Antimicrobial Susceptibility Testing (EUCAST) meningitis breakpoints18.
Incidence rates (IR) were calculated based on the population estimates provided by the Central Statistics Office and expressed as the number of serotyped isolates from cases per 100,000 population (/100,000) per epidemiological year (July-June). The population estimates for those ≥65 years of age in Ireland have increased by 33% over the 10-year period from 471,021 to 625,449, and the results were calculated accordingly to allow for this. The isolates were also categorised according to vaccine-associated serotypes: PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, 23F); the additional serotypes only included in PCV13, i.e. PCV13-7 serotypes (1, 3, 5, 6A, 7F, 19A); the PPV23 serotypes not covered in either of the PCV vaccines, i.e. PPV23-PCV13 (2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, 33F); non-vaccine associated serotypes (NVT) and non-typable isolates. Age-, vaccine- and non-vaccine serotype- specific IR were calculated and pre-vaccine (2007/08) and post-vaccine (2016/17) rates were compared. Incidence rate ratios (IRR) and 95% confidence intervals (95%CI) were calculated using online software (https://www.medcalc.org). A two-tailed p-value of ≤0.05 was considered to be statistically significant.
Results
During the ten years, a total of 1,578 culture positive isolates from adults ≥65 years of age were typed. Table 1 outlines the total number, incidence rate and the aggregated data per vaccine-associated serotype group based on the annual population estimate for the increasing age group ≥65 years of age. The total number of isolates typed has increased from 135 (28.66/100,000) in 2007/08 to 190 (30.38/100,000) in 2016/17. More isolates were received during the winter and early spring months and corresponded with the busiest influenza periods. The PCV7-associated serotypes fell by 85% in adults ≥65 years after the vaccine was introduced to the paediatric schedule in 2008 (IRR=0.11, 95%CI: 0.05-0.22, p<0.0001). There was a decline in all seven serotypes associated with PCV7.
Table 1: Incidence and number of serotypes from adults aged ≥65 years of age in Ireland with invasive pneumococcal disease, according to vaccine serotypes, 2007/08 to 2016/17.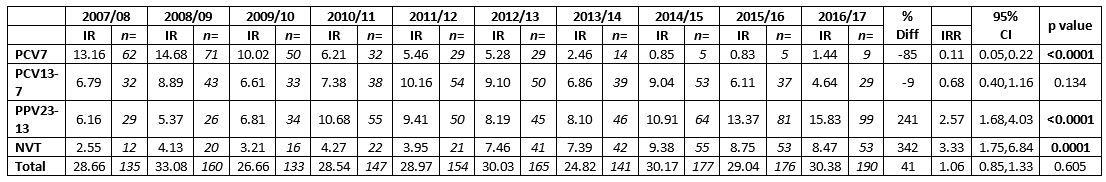 7-valent pneumococcal conjugate vaccine serotypes (PCV7), 13-valent pneumococcal conjugate vaccine serotypes not in PCV7 (PCV13-7) and 23-valent polysaccharide vaccine types not included in PCV13 (PPV23-PCV13) denote the serotypes in each vaccine-type group as listed. Non-vaccine types (NVT) denote all other serotypes not included in the PCV, PPV and non-typable isolates.
7-valent pneumococcal conjugate vaccine serotypes (PCV7), 13-valent pneumococcal conjugate vaccine serotypes not in PCV7 (PCV13-7) and 23-valent polysaccharide vaccine types not included in PCV13 (PPV23-PCV13) denote the serotypes in each vaccine-type group as listed. Non-vaccine types (NVT) denote all other serotypes not included in the PCV, PPV and non-typable isolates.
IR denotes the incidence rate of typed isolates per 100,000 population per epidemiological year.
n= number of isolates typed per epidemiological year.
Incidence Rate Ratio (IRR) denotes the ratio between the incidence rate of typed isolates in the epidemiological year 2016/17 in comparison to the start of surveillance in 2007/08 (pre PCV-vaccine).
95% CI denotes the 95% confidence interval of the IRR.
P value of <0.05 denotes a significant difference between two incidence rates (highlighted in bold text)
Figure 1: Number of penicillin susceptible and non-susceptible isolates causing invasive pneumococcal disease from adults aged ≥65 years of age in Ireland, from 2007/08 to 2016/17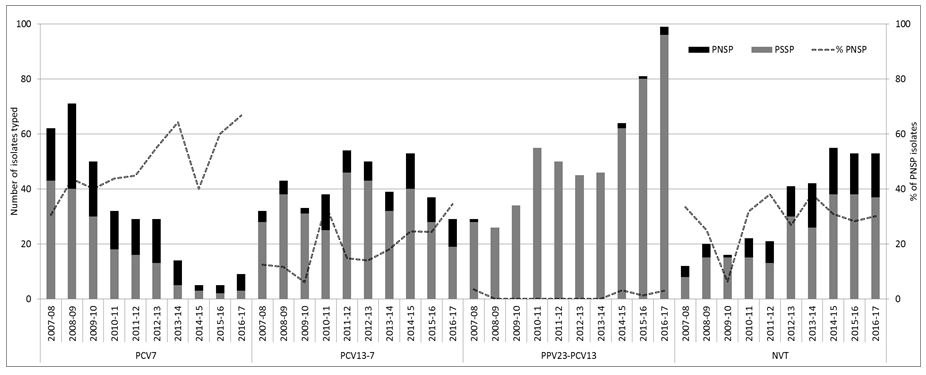 7-valent pneumococcal conjugate vaccine serotypes (PCV7), 13-valent pneumococcal conjugate vaccine serotypes not in PCV7 (PCV13-7) and 23-valent polysaccharide vaccine types not included in PCV13 (PPV23-PCV13) denote the serotypes in each vaccine-type group as listed. Non-vaccine types (NVT) denote all other serotypes not included in the PCV, PPV and non-typable isolates.
7-valent pneumococcal conjugate vaccine serotypes (PCV7), 13-valent pneumococcal conjugate vaccine serotypes not in PCV7 (PCV13-7) and 23-valent polysaccharide vaccine types not included in PCV13 (PPV23-PCV13) denote the serotypes in each vaccine-type group as listed. Non-vaccine types (NVT) denote all other serotypes not included in the PCV, PPV and non-typable isolates.
% PNSP represents percentage of the total number of isolates that were penicillin-non susceptible pneumococci (PNSP) per epidemiological year.
Following the decline of PCV7 serotypes there was a subsequent increase in PCV13-7 serotypes, from 6.79/100,000 in 2007/08 to a peak of 10.16/100,000 in 2011/12, this fell subsequently to 4.64/100,000 in 2016/17, (IRR=0.68, 95%CI:0.40-1.16, p=0.134), corresponding to a 9% decrease in isolate numbers. There were significant declines in three of the serotypes associated with PCV13-7, serotypes 1, 6A and 7F (n=0 for all three; p=0.046, 0.010 and 0.001, respectively). However, serotype 3 (IRR=1.08, 95%CI: 0.37-3.33, p=0.882) and serotype 19A (IRR=1.59, 95%CI: 0.69-3.99, p=0.248) have remained high in this population and consequently there was only a minor decline in the overall numbers of PCV13-7 serotypes (IRR=0.68, 95%CI: 0.40-1.16, p=0.134).
There was over a 2-fold increase in serotypes solely associated with the 23-valent polysaccharide vaccine (PPV23-PCV13) during the past ten years IRR=2.57, 95%CI: 1.68-4.03, p<0.0001), mainly due to increases in serotypes 8 (IRR=5.77, 95%CI: 1.73-19.23, p=0.0043), 22F (IRR=3.39, 95%CI:1.15-10.01, p=0.0272), 12F (IRR=2.11, 95%CI: 0.76-5.85, p=0.1521), 33F (IRR=2.13, 95%CI: 0.84-5.41, p=0.1105) and 9N (IRR=1.63, 95%CI: 0.62-4.29, p=0.3212).
There was also more than a 3-fold increase in non-vaccine types (NVTs) in the past ten years (IRR=3.33/100,000, 95%CI: 1.75-6.84, p=0.0001), especially serotypes 15A (IRR=6.78, 95% CI: 0.86-53.50, p=0.0695), 23A (IRR=2.26, 95%CI: 0.24-21.72, p=0.4803) and 35B (IRR=1.51, 95%CI:0.38-6.02, p=0.5624). Of note, 54% of serotype 15A (n=33/60) and 79% of serotype 35B (n=38/48) have had reduced susceptibility to penicillin over the ten years.
The remainder of penicillin non-susceptible pneumococci (PNSP) tended to be the serotypes included in the PCV7/13 vaccines (Figure 1). As a result, there was a reduction in the number of PNSP isolates in the PCV7 serotype group. However, the number of PNSP isolates within the PCV13-7 group has tended to fluctuate. Most of this fluctuation was associated with serotype 19A, which is typically associated with resistance (45% of the 19A). There was very little resistance associated with PPV23-PCV13 serotypes despite the significant increase in these serotypes in recent years (as illustrated in Figure 1).
Discussion
From the data presented here, it is clear that IPD is still responsible for an increasing number of invasive infections in the older adult population in Ireland, despite the positive impact of PCVs. Although overall the number of PCV7 and PCV13 serotypes has declined, some PCV13 serotypes have persisted (serotype 3 and 19A) in this patient cohort. Furthermore, there is clear evidence that the non-PCV13 serotypes covered in the adult PPV23 vaccine (PPV23-PCV13) and NVTs are increasing rapidly and are likely to erode the positive impact in reducing the burden of IPD in adults that was achieved through paediatric vaccination.
Two options available for attempting to reduce the incidence of invasive infection include vaccination of adults ≥65 years of age directly with PCV13 and/or improving PPV23 vaccine uptake rates. Firstly, a large clinical trial (CAPiTA) from the Netherlands found that the number of PCV13-associated IPD cases was lower after vaccination, with efficacy rates of 76% and 49% for PCV13 serotypes and all serotypes, respectively19. The administration of PCV13 may further reduce the incidence of these serotypes and overall IPD in the older adult population, particularly when serotypes 3 and 19A have remained predominant serotypes circulating in the Irish population. Arising from these findings, the United States (US) introduced one dose of PCV13 prior to administration of PPV23 in 2014 to further reduce the burden of IPD in adults20. This is despite the fact that the US had already seen a 58% decline of PCV13-7 serotypes as a result of PCV13 paediatric vaccination3. Further studies have indicated that PCV13 prior to PPV23 in patients ≥70 years of age elicits a higher antibody response to the PCV13 serotypes than with PPV23 alone21. However, more time will be needed to assess the potential benefits to these changes in vaccine policies.
Recent findings from a European collaborative pooled analysis (SpIDnet group) found that although the PPV23 vaccine effectiveness estimate against PPV23-serotype IPD was lower than expected (43%), vaccinating the elderly with PPV23 may still prevent at least 25% of cases of IPD22.The results indicated that the vaccine effectiveness also varied depending on serotype, with some vaccine serotypes having a higher effectiveness (serotype 9N = 55%, CI:15-76%) than others (serotype 3 = -2%, CI:-48-30%). It is also worth noting that serotype 9N along with other PPV23 serotypes are continually increasing in this population in Ireland. It is likely that improving PPV23 uptake the adult population in Ireland would still provide better protection in this population.
Whether PPV23 is administered directly to adults with/without a prior dose of PCV13, the vaccination uptake rate of PPV23 among adults aged ≥65 years needs to be improved from 36% as of 2013 in Ireland to reduce the burden of IPD13. Previous studies in Ireland reported that around 80% of older persons would consider immunisation with pneumococcal vaccines from their doctor if contacted23. Alternatively, a more pro-active campaign such as recognising the opportunity to vaccinate while attending hospitals or increased national advertising similar to the seasonal influenza campaign, which had an uptake of 53-70% may improve uptake24. Furthermore, clinical data that suggests dual immunisation with pneumococcal and influenza vaccines concomitantly reduces the cases of pneumonia and overall mortality in older adults may be worth considering25. This is important as there is clear evidence to indicate that IPD incidence rates, severity of clinical presentation and case fatality ratio increases during the influenza period26, 27. The number of cases of IPD during the influenza season in Ireland also merits dual vaccination being considered.
This paper highlights that the number of PPV23-PCV13 and non-vaccine serotypes are increasing rapidly in Ireland and this is partly eroding the positive impact due to the overall decline in IPD in the adult age group following the introduction of PCVs in children. Increased vaccine uptake should be addressed to reduce the burden of disease in this growing population.
Conflicts of Interest Statement
HH has recently received research funds from Astellas and Pfizer, and has received lecture and other fees from Cepheid and Astellas. MCo and MMcE have received funding support from Pfizer. Additional funding has been provided through an unrestricted research grant from Pfizer (Ireland). The funders had no role in the collection, analysis, interpretation of data or in the writing of and decision to submit the article for publication.
Acknowledgements
We would like to thank the clinical laboratories for submitting isolates for typing and the Department of Public Health for continual surveillance. This project is funded by the Royal College of Surgeons Ireland, Temple Street Children's University Hospital, Health Protection Surveillance Centre and previously through Pfizer Ireland (unrestricted grant). We are also partially supported by the ECDC (SpIDnet project) and the European Commission (Horizon 2020).
Corresponding Author
Dr. Mary Corcoran,
The Irish Pneumococcal Reference Laboratory,
Irish Meningitis and Sepsis Reference Laboratory,
Temple Street Children’s University Hospital,
Temple Street,
Dublin 1,
Ireland.
Telephone: +3531-878-4854
Email: [email protected]; [email protected]
References
1. Gierke RLM, Beall B, Pilishivili T. CDC.Vaccine Preventable Disease Surveillance Manual Pneumococcal: Chapter 11.1. 2017 [Internet] Available from: https://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.pdf.
2. Savulescu C, Krizova P, Lepoutre A, Mereckiene J, Vestrheim DF, Ciruela P Ordobas M, Guevara M, McDonald E, Morfeldt E, Kozakova J, Varon E, Cotter S, Winje BA, Munoz-Almagro C, Garcia L, Castilla J, Smith A, Henriques-Normark B, Celentano LP, Hanquet G on behalf of the SpIDnet group. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study. The Lancet Respiratory Medicine. 2017 2017/04/03.
3. Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, Miller L, Scherzinger K, Thomas, A, Farley, MM, Zell, ER, Taylor TH, Pondo T, Rodgers L, McGee L, Beall, Bernard, Jorgensen, James H. Whitney, Cynthia G. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. The Lancet Infectious Diseases. 2015 2015/11/27;15(3):301-9.
4. Debby BD, Mitchell JS, Amos A, Samira M, Rotem E, Shiri NV, Schwartz D, Porat N, Kotlovsky T, Polivkin N, Weinberg I, Lazary A, Ohana N, Dagan R. Persistence and Complex Evolution of Fluoroquinolone-Resistant Streptococcus pneumoniae Clone. Emerging Infectious Disease journal. 2014;20(5):799.
5. Health Protection Surveillance Centre. Immunisation uptake report for Ireland - A report by the Health Protection Surveillance Centre - Q2 2016. 2016 [Internet]; Available from: http://www.hpsc.ie/A-Z/VaccinePreventable/Vaccination/ImmunisationUptakeStatistics/Immunisationuptakestatisticsat12and24monthsofage/AnnualReports/File,15662,en.pdf).
6. Health Protection Surveillance Centre. Streptococcus pneumoniae (invasive) in Ireland, 2017 - Annual Epidemiological Report [Internet]; Available http://www.hpsc.ie/a-z/vaccinepreventable/pneumococcaldisease/publications/annualreportsoninvasivepneumococcaldisease/Invasive%20pneumococcal%20disease%20AER%202017.pdf
7. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MPE, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. The Lancet Infectious Diseases. 2015 2015/06/02;15(5):535-43.
8. van der Linden M, Falkenhorst G, Perniciaro S, Imohl M. Effects of Infant Pneumococcal Conjugate Vaccination on Serotype Distribution in Invasive Pneumococcal Disease among Children and Adults in Germany. PLoS ONE. 2015;10(7):e0131494.
9. Regev-Yochay G, Paran Y, Bishara J, Oren I, Chowers M, Tziba Y, Istomin V, Weinberger M, Miron D, Temper V, Rahav, G, Dagan, RonEarly impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: A nationwide surveillance study. Vaccine. 2015;33(9):1135-42.
10. Corcoran M, Vickers I, Mereckiene J, Murchan S, Cotter S, Fitzgerald M, Fitzgerald M, McElligott M, Cafferkey M, O'Flanagan, D, Cunney R, Humphreys H. The epidemiology of invasive pneumococcal disease in older adults in the post-PCV era. Has there been a herd effect? Epidemiology and Infection. 2017;145(11):2390-9.
11. National Immunisation Advisory Committee. Chapter 3 Immunisation of Immunocompromised Persons. [Internet]. Available from: https://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter3.pdf.
12. Central Statistics Office. Women and Men in Ireland - Health. 2016 [Internet]. Available from: http://www.cso.ie/en/releasesandpublications/ep/p-wamii/womenandmeninireland2016/health/.
13. Giese C, Mereckiene J, Danis K, O'Donnell J, O'Flanagan D, Cotter S. Low vaccination coverage for seasonal influenza and pneumococcal disease among adults at-risk and health care workers in Ireland, 2013: The key role of GPs in recommending vaccination. Vaccine. 2016;34(32):3657-62.
14. Public Health England. Pneumococcal Polysaccharide Vaccine (PPV) coverage report, England, April 2015 to March 2016. 2016 [Internet]. 2017. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/540290/hpr2416_ppv.pdf
15. Fedson, DS, Nicolas-Spony L, Klemets P, van der Linden M, Marques A, Salleras L, Samson SI. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe, Expert Review of Vaccines. 2011 10(8) 1143-1167.
16. Bergh C, Fall K, Udumyan R, Sjöqvist H, Fröbert O, Montgomery S. Severe infections and subsequent delayed cardiovascular disease. European Journal of Preventive Cardiology. 2017 2017/10/20:1-9.
17. Vickers I, O'Flanagan D, Cafferkey M, Humphreys H. Multiplex PCR to determine Streptococcus pneumoniae serotypes causing otitis media in the Republic of Ireland with further characterisation of antimicrobial susceptibilities and genotypes. European Journal of Clinical Microbiology & Infectious Diseases. 2011;30(3):447-53.
18. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 7.1, valid from 2017-03-10. [Internet]. 2017. Available from: http://www.eucast.org/clinical_breakpoints/.
19. Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AMM, Sanders EAM, Verheij TJM, Patton Ml, McDonough A, Moradoghli-Haftvani A, Smith H, Mellelieu T, Pride MW, Crowther G, Schmoele-Thoma B, Scott, DA, Jansen KU, Lobatto R, Oosterman B, Visser, N, Caspers E, Smorenburg A, Emini EA, Gruber WC, Grobbee DE. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. New England Journal of Medicine. 2015;372(12):1114-25.
20. Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report (MMWR). 2014;37(63):822-5.
21. Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini, EA, Gruber WC, Schmoele-Thoma Beate. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2015;31(35):3585-93.
22. SpIDnet Group. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in European adults aged ≥65 years and above: results of spidnet/i-move+ multicentre study (2012-2016). ISPPD; 2018.
23. Bedford D IG, White M, Parsons B, Foyle D, Howell F, Corcoran R. The Acceptability of Pneumococcal Vaccine to Older Persons in Ireland. Irish Medical Journal. 2000;93(2):48-9.
24. Chaintarli K, Barrasa A, Cotter S, Mereckiene J, O’Donnell J, Domegan L. Decrease in the Uptake of Seasonal Influenza Vaccine in Persons Aged 65 Years and Older In Ireland since the 2009 Influenza A (H1N1) Pdm09 Pandemic. Irish Medical Journal. 2017; 110(9).
25. Zhang YY, Tang XF, Du CH., Wang, BB., Bi, ZW, Dong, BR. Comparison of dual influenza and pneumococcal polysaccharide vaccination with influenza vaccination alone for preventing pneumonia and reducing mortality among the elderly: A meta-analysis. Human Vaccines & Immunotherapeutics 2016;12, 3056–3064.
26. Burgos J, Larrosa MN, Martinez A, Belmonte J, González-López J, Rello J, Pumarola T, Pahissa A, Falco V. Impact of influenza season and environmental factors on the clinical presentation and outcome of invasive pneumococcal disease. European Journal of Clinical Microbiology & Infectious Diseases.2015;34(1):177-186.
27. Weinberger DM, Harboe ZB, Viboud CV, Krause TG, Miller M, Mølbak K. Konradsen, HB. Pneumococcal disease seasonality: incidence, severity and the role of influenza activity. European Respiratory Journal, 2014; 43(3):833-841.
P894
