Kawasaki Disease – A Review of Treatment and Outcomes in an Irish Paediatric Cohort 2010-14.
Flinn AM1, Gavin PJ1, McMahon CJ1,2, Oslizlok P1, Butler KM1,2
1Our Lady’s Children’s Hospital, Dublin, Ireland
2University College Dublin, Ireland
Abstract
Diagnosis of Kawasaki Disease (KD) can be challenging due to lack of a diagnostic test, and some children present with ‘incomplete’ KD when not all diagnostic criteria are met. Treatment with intravenous immunoglobulin (IVIG) and aspirin reduces the risk of coronary artery complications. There is sub-group of patients who are resistant to IVIG/aspirin therapy and are at increased risk of complications. Recent evidence suggests that additional treatment of this high-risk group with corticosteroids is beneficial in reducing this risk. We examine the treatment and coronary artery outcomes, by retrospective review of medical records, of a cohort of 32 paediatric patients with KD admitted to a single Irish tertiary centre from January 2010-December 2014. Twenty-eight percent of patients (9/32) had an incomplete diagnosis of KD; these patients received IVIG later compared to those with a complete KD diagnosis. 15/32 (47%) had abnormal echocardiogram findings in the acute phase, 8/32 (25%) had echocardiogram abnormalities at 6-week follow-up, and 4/32 (12.5%) had persisting abnormalities. This study highlights the potential for adverse outcome in KD, the difficulty in diagnosis in ‘incomplete’ cases, and the need to identify children at higher risk for adverse outcome where adjunctive therapies would be most beneficial.
Introduction
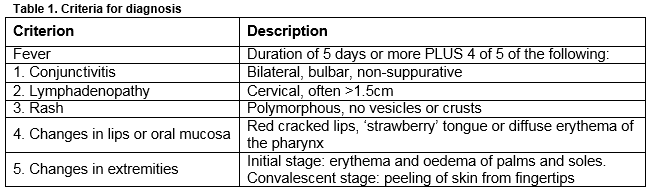
Kawasaki Disease (KD) is an acute self-limiting systemic vasculitis, predominantly affecting medium-sized arteries with a predilection for the coronary arteries. Primarily affecting children less than 5 years, it is the most common cause of acquired cardiac disease in children in the developed world1. A study from Ireland from 1996-2000 demonstrated a rate of 15.2 per 100,000 children less than 5 years, one of the highest in Europe2. An increasing incidence worldwide has been observed3 but it is unclear whether this is due to heightened awareness and thus increased diagnosis or true increasing incidence. Diagnosis is made using clinical criteria (Table 1); there are no diagnostic tests currently available, posing a challenge to clinicians, particularly when not all criteria are met, so called “incomplete” KD. Coronary artery aneurysms (CAA) develop in 15-25% of untreated children and can rupture or progress to myocardial ischaemia or infarction, causing sudden death1,4.
Treatment with intravenous immunoglobulin (IVIG) and aspirin (ASA) within 10 days of onset of symptoms reduces the incidence of CAA to less than 5% and are accepted as standard of care1, 5-8. However, up to 10%-20% of patients are resistant to initial IVIG therapy, characterized by persistence of fever and/or clinical signs1,9,10. This subgroup of patients are at increased risk of complications and require additional, as yet non-standardised, therapy which may include further IVIG, corticosteroids or other immunosuppressive agents such as infliximab1,9,10. Questions remain as to how to identify IVIG-resistant KD cases and target for additional therapy at presentation10. Scoring systems used to identify this high-risk group in Japanese children with KD lack sensitivity in a non-Japanese population11. The aim of this study was to review a cohort of children diagnosed with KD over a five-year period, examining the treatment regimens used, and coronary artery outcomes.
Methods
This was a retrospective review of the medical records of children admitted to Our Lady’s Children’s Hospital Crumlin (OLCHC) with a diagnosis of KD from January 2010 - December 2014. Patients were identified using the Infectious Diseases Departmental database and the OLCHC Hospital In-Patient Enquiry (HIPE) database. Data collected included age at diagnosis, gender, diagnostic categorisation as complete or incomplete, echocardiogram findings during admission and at six-week follow-up, and details of therapy received. Diagnosis was ‘complete’ if the criteria were fulfilled as described in Table 1, or ‘incomplete’ when all criteria were not met, but the diagnosis was still considered to be KD by the treating clinician, or if there were abnormal findings consistent with KD on echocardiogram. A Consultant Paediatric Cardiologist reported all echocardiogram findings. An echocardiogram was considered abnormal if any of the following were present: coronary artery dilation or aneurysm formation, acute valvular dysfunction or myocardial dysfunction suggestive of myocarditis. Given the retrospective non-interventional nature of this review, specific informed consent for chart review was not required. Statistical analysis was done using the Mann-Whitney Test.
Results
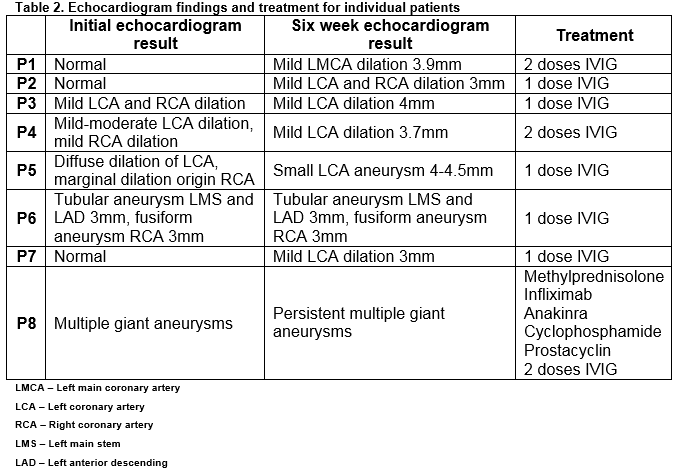
Thirty-two patients, 17 males, with median age at diagnosis of 28 months (range 2-132 months) were identified. Seven patients (22%) were less than 12 months of age. Twenty-three (72%) patients were categorised as complete KD; nine (28%) were incomplete. Fifteen (47%) patients, including 6 with incomplete KD, had abnormalities on echocardiogram during the acute phase of the illness: 13 (41%) on their initial echocardiogram and two (6%) who, despite having a normal initial echocardiogram, developed later abnormality during the acute phase of the illness. Eight patients (25%) had an abnormal echocardiogram at six-week follow-up, three of whom had a previously normal echocardiogram (Table 2). Overall 4/32 patients (12.5%) had persisting echocardiographic abnormalities. Four patients with resolved abnormalities had a normal echocardiogram after a median of 9.5 months follow-up. Two of these patients had incomplete KD at diagnosis. The incidence of echocardiograph abnormalities at six-week follow-up was similar in incomplete (3/9, 33%) and complete KD (5/23, 22%) patients.
Thirty-one patients received at least one dose of IVIG (2g/kg/dose), 30 of whom received concomitant ASA (50-100mg/kg/day). ASA was withheld in one patient because of thrombocytopaenia. One patient was afebrile at presentation three weeks after illness onset and did not receive IVIG or high-dose ASA. Among 29 patients with defined timing of symptom onset, the first dose of IVIG was given a median of 6 days after symptom onset (range 4-23) and 3 days (range 1-14) after hospital admission. Twenty-two (76%) patients received the first dose of IVIG within 10 days of symptom onset, 6/22 (27%) had an abnormal echocardiogram at six-week follow-up compared to 2/6 (29%) who received the first dose of IVIG ≥10 days after symptom onset. Time from symptom onset to administration of IVIG was not significantly different among the 8 patients with an abnormal six-week echocardiogram (median, 9 days) and patients with a normal six-week echocardiogram (median, 6 days) (p=0.48). IVIG was given a median of 12 days after symptom onset among patients with incomplete KD compared to 6 days among patients with complete KD (p=0.02).
Four patients (three complete KD, one incomplete KD) received a variety of corticosteroid regimens. Three were admitted to the intensive care unit. All corticosteroid recipients had an abnormal echocardiogram prior to corticosteroid use. Two patients received further immunosuppressive therapy with (one with infliximab and one with infliximab, anakinra and cyclophosphamide). At follow-up, two patients had normal echocardiograms, one had persistent multiple giant CAA (with some improvement) and the result for one patient was unknown.
Discussion
In this cohort from a single tertiary paediatric centre, KD occurred predominantly in children less than 5 years old, consistent with the literature. However, the proportion of young infants with KD was high, with seven children (22%) less than 12 months old and one as young as 2 months at diagnosis. One in four patients failed to fulfill diagnostic criteria and were classified as incomplete KD. A similar increase in the proportion of incomplete KD cases has recently been reported12. Our patients with incomplete KD received treatment later compared to patients with complete KD, reflecting the difficulty in recognition and the need to maintain a high index of suspicion for this condition. Although a higher risk of abnormalities on six-week echocardiogram has been reported by others in incomplete KD patients, the incidence of abnormalities was similar in our cohort of KD patients13, 14.
In this small cohort of KD patients, the incidence of coronary artery abnormalities acutely (47%) and at follow-up (25%) was striking. That over 20% received IVIG beyond 10 days of illness may have been a contributing factor. It is well known that CAA occur in 15-25% of untreated individuals, but less data is available regarding the prevalence of coronary lesions in the acute phase of the illness. Kuwabara et al reported that cardiac abnormalities at first hospital presentation were detected in 4.25% of 26,691 KD patients in Japan15.
Seven of eight patients with abnormal follow-up echocardiograms had aneurysms defined as ‘small’1 and while the prognosis for this group is more favourable16,17, concerns exist that coronary remodelling in regressed aneurysms may be associated with atherosclerotic lesions later in life18. Five of the eight patients received a single dose of IVIG.
Four (12.5%) patients, three of whom were refractory to IVIG therapy, received corticosteroids. All four had abnormal echocardiogram findings and other risk factors for poor outcome at presentation10. Earlier initiation of adjunctive corticosteroid therapy in Japanese children with high-risk factors at presentation reduces coronary artery complications19, 20. Whether corticosteroid and IVIG therapy at presentation rather than waiting to determine IVIG responsiveness might alter the outcome for our patients is unclear. Some experts have suggested that primary corticosteroid and IVIG therapy be considered for KD in young infants (<6 or 12 months old) or with coronary artery abnormalities at presentation, subgroups known to be at higher risk for adverse outcome10, 21. However, in our KD cohort, three of 8 of patients with demonstrable coronary artery abnormalities at six-week follow-up had normal echocardiograms at presentation and would not have been considered candidates for primary corticosteroid therapy (Table 2). At present, for the majority of patients with KD, corticosteroids remain second line rescue therapy in cases of severe or resistant disease20, 22.
Limitations of this study include its retrospective nature and small cohort size. OLCHC is the tertiary paediatric cardiology centre in Ireland and some of the patients were transferred from regional centers because of more severe disease. This likely biased the cohort toward the more severe end of the disease spectrum resulting in a higher incidence of CAA than in the general KD population. Some cases may have been missed due to under-reporting in the HIPE database, although recent assessment of HIPE coding has shown significant improvement23, and this risk was reduced by cross-referencing with the Infectious Diseases Departmental database. Nonetheless the study shows the potential for adverse outcome in KD and, with almost 30% ‘incomplete’ the difficulty posed in its early recognition. It highlights the continuing need for a diagnostic test to shorten the window between symptom onset and treatment and identify the subset of children at higher risk for adverse outcome where adjunctive therapies would be most beneficial. Clinicians must maintain a high index of suspicion for KD in febrile children presenting with persisting fever and rash even when all of the criteria are not fulfilled i.e. “incomplete” KD.
Continued collaborative research is needed to optimise therapeutic regimens and identify subgroups of patients at highest risk of adverse outcomes. To achieve better clinical outcomes for all patients with KD, further research is required into understanding possible genetic predisposition and to unravel the mystery of the underlying pathogen or pathogens at large. A nationwide database of paediatric patients with KD to provide updated information on the national rate of hospitalization associated with KD among children <18 years in Ireland and to gather information based on larger patient numbers with regard to treatment practices and coronary artery outcomes would help in this regard.
Conflicts of Interest
The authors confirm that there are no conflicts of interest.
Correspondence
Dr Aisling Flinn, Our Lady’s Children’s Hospital, Dublin, Ireland
Email: [email protected]
References
1. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004; 110:2747-71.
2. Lynch M, Holman RC, Mulligan A, Belay ED, Schonberger LB. Kawasaki syndrome hospitalizations in Ireland, 1996 through 2000. Pediatr Infect Dis J 2003; 22:959-63.
3. Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child 2015; 100:1084-8.
4. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996; 94:1379-85.
5. Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, Takahashi M, Bierman FZ, Karchmer AW, Wilson W, Rahimtoola SH, Durak DT, Peter G. Diagnosis and therapy of Kawasaki disease in children. Circulation 1993; 87:1776-80.
6. Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics 1995; 96:1057-61.
7. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr 1988; 113:290-4.
8. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP, Mason WH, HC Meissner, Rowley AH, Shulman ST, Reddy V, Sundel RP, Wiggins JW, Colton T, Melish ME, Rosen FS. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 1991; 324:1633-9.
9. Brogan PA, Bose A, Burgner D, Shingadia D, Tulloh R, Michie C, Klein N, Booy R, Levin M, Dillon MJ. Kawasaki disease: an evidence based approach to diagnosis, treatment, and proposals for future research. Arch Dis Child 2002; 86:286-90.
10. Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Child 2014; 99:74-83.
11. Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, Atz AM, Printz BF, Baker A, Vetter VL, Newburger JW. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr 2011; 158:831-5.e3.
12. Maric LS, Knezovic I, Papic N, Mise B, Roglic S, Markovinovic L, Tesovic G. Risk factors for coronary artery abnormalities in children with Kawasaki disease: a 10-year experience. Rheumatology International 2015; 35:1053-8.
13. Ha KS, Jang G, Lee J, Lee K, Hong Y, Son C, Lee J. Incomplete clinical manifestation as a risk factor for coronary artery abnormalities in Kawasaki disease: a meta-analysis. Eur J Pediatr 2013; 172:343-9.
14. Song D, Yeo Y, Ha K, Jang G, Lee J, Lee K, Son C, Lee J. Risk factors for Kawasaki disease-associated coronary abnormalities differ depending on age. Eur J Pediatr 2009; 168:1315-21.
15. Kuwabara M, Yashiro M, Kotani K, Tsuboi S, Ae R, Nakamura Y, Yanagawa H, Kawasaki T. Cardiac lesions and initial laboratory data in Kawasaki disease: a nationwide survey in Japan. J Epidemiol 2015; 25:189-93.
16. Patel AS, Bruce M, Harrington W, Portman MA. Coronary artery stenosis risk and time course in Kawasaki disease patients: experience at a US tertiary pediatric centre. Open Heart 2015; 2:e000206.
17. Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics 1979; 63:175-9.
18. Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: vascular wall morphology and function. Heart 2000; 83:307-11.
19. Chen S, Dong Y, Yin Y, Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart 2013; 99:76-82.
20. Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, Kato T, Hara T, Hamaoka K, Ogawa S, Miura M, Nomura Y, Fuse S, Ichida F, Seki M, Fukazawa R, Ogawa C, Furuno K, Tokunaga H, Takatsuki S, Hara S, Morikawa A. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet 2012; 379:1613-20.
21. Shulman ST. Early steroid therapy reduces Kawasaki disease coronary complications. J Pediatr 2017; 182:401-4.
22. Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, Atz AM, Li JS, Takahashi M, Baker AL, Colan SD, Mitchell PD, Klein GL, Sundel RP. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med 2007; 356:663-75.
23. Brabazon ED, Sheridan A, Finnegan P, Carton MW, Bedford D. Under-reporting of notifiable infectious disease hospitalizations: significant improvements in the Irish context. Epidemiol Infect 2015; 143:1166-74.
(P691)