Meconium Ileus in Two Irish Newborns: The Presenting Feature of Cystic Fibrosis
A.Smith1 , E. Ryan1, D. O’Keeffe2, D. O’Donovan1
1. Department of Neonatology, University Hospital, Galway
2. Department of Radiology, University Hospital Galway
Abstract
Introduction
Meconium Ileus (MI) is the presenting feature of CF in approximately 10-15% of cases. This report outlines the clinical presentation, imaging and management of two neonates with MI and subsequent diagnosis of Cystic Fibrosis (CF).
Methods
A retrospective chart review was performed to evaluate the clinical course of two neonates with MI.
Results
Case 1 and 2 presented clinically with signs of abdominal obstruction. Subsequent laparotomies confirmed MI. MI is strongly associated with CF and CF is the most common genetically inherited disease in Ireland. Genetic testing was positive for a homozygous ∆ F508 mutation in both case 1 and 2, securing a diagnosis of MI secondary to CF.
Conclusion
Our cases highlight that all infants born in Ireland with MI should be considered as CF positive until proven otherwise.
Introduction
Cystic Fibrosis (CF) is the most common genetically inherited disease in Ireland1. Approximately 1/ 2,300 infants per year are born with CF in Ireland2. Newborn bloodspot screening (NBS) screening for CF was introduced to Ireland in 20113. NBS screening for CF is associated with improved lung function, nutritional status and increased survival into early adulthood4. Therefore early recognition and management of this chronic condition is vital to ensuring optimal patient management.
Intestinal obstruction is the most common surgical emergency in the neonatal period and a frequent cause for admission to the neonatal intensive care unit5,6. The four cardinal features of neonatal intestinal obstruction are maternal polyhydramnious, bilious aspirates/vomits, abdominal distension and failure to pass meconium within the first 24 hours of life6. Prompt recognition of the signs and symptoms of intestinal obstruction with rapid escalation of management is vital to promote the best possible outcome. The differential diagnosis of neonatal intestinal obstruction includes atresia, malrotation, stenosis, Hirschsprung disease and meconium ileus (MI). MI is estimated to account for 20% of neonatal intestinal obstruction5. This report outlines the clinical presentation, imaging and management of two neonatal patients with meconium ileus and subsequent diagnosis of cystic fibrosis.
Case 1
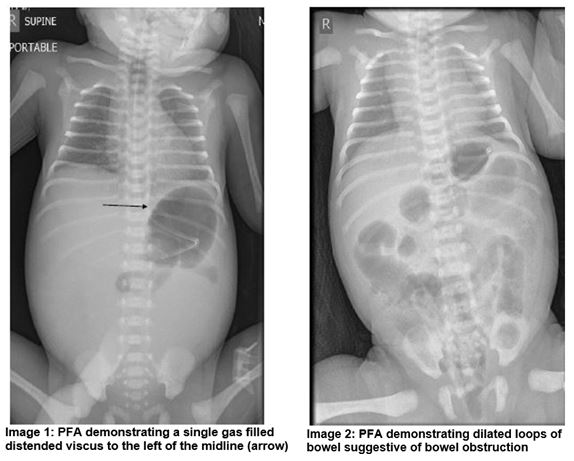
A dichorionic diamniotic (DCDA) female twin was born at 33+2 weeks gestation and birth weight of 2.37kg. A distended abdomen was noted from the delivery suite, the anus was patent. A nasogastric tube (NGT) was passed and yellow coloured fluid was aspirated. A plain film of abdomen (PFA) demonstrated that the stomach was distended with gas and a single gas filled distended viscus to the left of the midline was noted which raised the possibility of an atresia (Image 1).The baby was transferred to a tertiary surgical centre where laparotomy confirmed meconium ileus (MI). A segmental volvulus resection was also performed. Post operatively an ileus developed requiring further surgical management and resection of adhesions with ileostomy formation.
Case 2
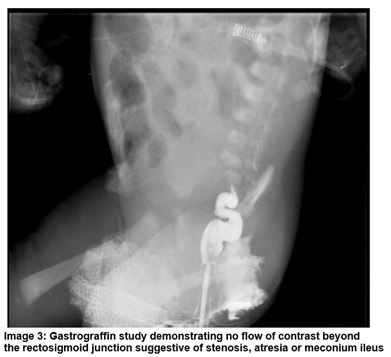
A male infant was born at 38+2 weeks gestation and birth weight of 3.42kg. Polyhydramnios was noted on antenatal scanning. On day one the infant had repeated episodes of vomiting in between feeds and had not passed meconium from birth. Abdominal distension was evident and following NGT placement there were bile stained aspirates. The anus was patent. A PFA demonstrated dilated loops of bowel and a Gastrograffin study showed no flow of contrast beyond the rectosigmoid junction (Image 2 and 3). The baby was transferred to a tertiary surgical centre where laparotomy confirmed MI. A bowel washout was performed and stoma formation was not required.
MI is virtually always associated with Cystic Fibrosis (CF). As such all Irish infants with MI are genetically screened for the 39 most common mutations in CF. Genetic testing was positive for a homozygous ∆ F508 mutation in both case 1 and 2, securing a diagnosis of MI secondary to CF.
Discussion
Meconium ileus is defined as a small bowel obstruction due to inspissated meconium within the terminal ileum7, 8. MI typically occurs in the neonatal period, however there have been rare reports of MI presenting up to 6 months of age 9. While essentially synonymous with CF, MI may also occur with pancreatic atresia or pancreatic duct stenosis10. MI is more common in white populations and affects males and females equally 10.
Antenatal ultrasound may reveal signs suspicious of MI including polyhydramnios, calcification, intra-abdominal cysts and hyperechoic bowel loops10, 11. Postnatally, radiological features of MI on plain radiograph may include dilated loops of bowel proximal to the obstruction, absence or paucity of air-fluid levels and a ‘bubbly’ appearance of the distended intestinal loops 10. Other imaging studies may include a contrast enema which may illustrate impacted meconium in the right colon or distal ileum.
MI may be complicated by volvulus, perforation of peritionitis12. Intrauterine perforation may lead to meconium peritionitis and peritoneal calcification may be visible on imaging postnatally 11. The differential diagnosis of MI includes meconium plug syndrome and total colonic Hirschsprung disease 10. The management of choice for uncomplicated MI is non-operative Gastrografin enema, which is successful in over 60% of cases 10, 12. Laparotomy with stoma formation or bowel resection with primary anastomosis is reserved for complicated cases or enema failure 12.
MI is the presenting feature of CF in approximately 10-15% of cases13. It is recognised that infants with CF who present with MI may have lower than expected blood Immunoreactive trypsinogen (IRT) levels which can result in a false negative CF NBS result14, 15. As such the National Newborn Screening Steering Committee advise that all infants presenting with MI should have blood sent for CF gene mutation analysis irrespective of CF NBS result, as was the case for the two infants discussed in this series.
Height and weight percentiles may be lower at 15 and 25 years of age for patients with CF and MI than those with CF without MI, however pulmonary function is comparable between the two groups7. A 2016 study reported that certain CF transmembrane conductance regular (CFTR) gene mutations posed a higher risk of MI 16. Utilising a MI prevalence (MIP) score Dupuis et al found that the G542X mutation conferred a high risk, the ∆ F508 mutation a moderate risk and the G551D mutation a low risk of developing MI respectively 16.
In conclusion, timely diagnosis of MI is essential to minimise morbidity and optimise patient outcome. This report highlights clinical features and imaging studies which will guide management of such patients. MI is strongly associated with CF and as such all infants with MI should be considered as CF positive until proven otherwise.
Conflicts of Interest Statement
The authors have no conflict of interest to declare.
Corresponding Author
Aisling Smith,
The Rotunda Hospital,
Parnell Square,
Dublin
Email: [email protected]
References
1. Cystic Fibrosis Ireland 2018 [Available from: https://www.cfireland.ie/.
2. HSE NNBSLTSCsUH. A Practical Guide to Newborn Bloodspot screening in Ireland. 2016(6th Edition).
3. HSE. A Practical Guide to Newborn Bloodspot Screening in Ireland. 2011.
4. Dijk FN, McKay K, Barzi F, Gaskin KJ, Fitzgerald DA. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same centre. Archives of disease in childhood. 2011;96(12):1118-23.
5. Vinocur DN, Lee EY, Eisenberg RL. Neonatal intestinal obstruction. AJR Am J Roentgenol. 2012;198(1):W1-10.
6. Juang D, Snyder CL. Neonatal bowel obstruction. Surg Clin North Am. 2012;92(3):685-711, ix-x.
7. Carlyle BE, Borowitz DS, Glick PL. A review of pathophysiology and management of fetuses and neonates with meconium ileus for the pediatric surgeon. Journal of pediatric surgery. 2012;47(4):772-81.
8. Copeland DR, St Peter SD, Sharp SW, Islam S, Cuenca A, Tolleson JS, Dassinger MS, Little DC, Jackson RJ, Kokoska ER, Smith SD. Diminishing role of contrast enema in simple meconium ileus. Journal of pediatric surgery. 2009;44(11):2130-2.
9. Baral V, Connett G. Acute intestinal obstruction as a presentation of cystic fibrosis in infancy. J Cyst Fibros. 2008;7(4):277-9.
10. Radiopaedia [Available from: https://radiopaedia.org/articles/meconium-ileus.
11. Chan KL, Tang MH, Tse HY, Tang RY, Tam PK. Meconium peritonitis: prenatal diagnosis, postnatal management and outcome. Prenatal diagnosis. 2005;25(8):676-82.
12. Rescorla FJ, Grosfeld JL. Contemporary management of meconium ileus. World journal of surgery. 1993;17(3):318-25.
13. Mushtaq I, Wright VM, Drake DP, Mearns MB, Wood CB. Meconium ileus secondary to cystic fibrosis. The East London experience. Pediatr Surg Int. 1998;13(5-6):365-9.
14. Heeley AF, Bangert SK. The neonatal detection of cystic fibrosis by measurement of immunoreactive trypsin in blood. Annals of clinical biochemistry. 1992;29 ( Pt 4):361-76.
15. del Ciampo IR, Oliveira TQ, del Ciampo LA, Sawamura R, Torres LA, Augustin AE, Fernandes MI. [Early manifestations of cystic fibrosis in a premature patient with complex meconium ileus at birth]. Revista paulista de pediatria : orgao oficial da Sociedade de Pediatria de Sao Paulo. 2015;33(2):241-5.
16. Dupuis A, Keenan K, Ooi CY, Dorfman R, Sontag MK, Naehrlich L, Castellani C, Strug LJ, Rommens JM, Gonska T. Prevalence of meconium ileus marks the severity of mutations of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. Genetics in medicine : official journal of the American College of Medical Genetics. 2016;18(4):333-40.
P901
