Palliative Subcutaneous Terbutaline Infusion in Severe Asthma
A Sahadevan, SJ Lane
Department of Respiratory Medicine, AMNCH, Tallaght, Dublin 24
Abstract
Continuous subcutaneous infusion of terbutaline (CSIT) has been shown to improve asthma control. As an adjunct to standard asthma pharmacotherapy, 3-12mg/day of CSIT can stabilise asthmatic symptoms, reduce hospitalisations and reduce corticosteroid need1,2. Asthmatics who demonstrate a wide diurnal variability in their peak-flows (>40%), termed brittle asthmatics tend to benefit the most from this therapy3. CSIT can have adverse effects and should only be used in specialist respiratory centres.
Introduction
A 56 year-old lady was admitted for palliation of severe asthma. She had a FEV1 of 70% (1.52L) with physiologic air trapping (RV 116%). She was on fluticasone propionate, salmeterol, oral theophylline, maintenance corticosteroids and montelukast. Her absence of atopy and a normal IgE count precluded anti-IgE (omalizumab) therapy. Bronchial thermoplasty was considered but excluded due to obesity. Despite observed pharmacotherapy, she remained symptomatic with chest tightness and wheeze. She demonstrated large diurnal peak-flow variability (47%). Asthma control test was 6 (poor symptomatic control). Continuous subcutaneous infusion of terbutaline (CSIT) has been shown to be beneficial as add-on therapy in uncontrolled asthmatics. Terbutaline infusions can stabilise asthma, reduce asthma-related hospital admissions, and reduce the corticosteroid burden1,2. A particular phenotype termed brittle asthmatics, defined by Turner and Ayres, appear to respond best to CSIT. Brittle asthmatics demonstrate wide variability in peak-flows (>40%) despite maximal pharmacotherapy.
Case Report
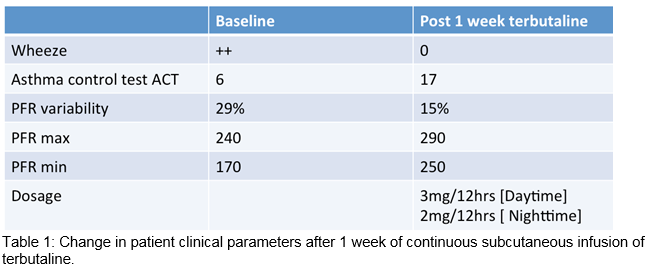
Our patient fit the phenotype of a brittle asthmatic. Pre-CSCIT we ensured normal electrolytes. A starting dose of terbutaline at 1.5mg/12 hours was initiated. This low dose was used to ensure tolerability and documentation of side effects related to both infusion device and medication. The terbutaline dose was then increased after 3 days to 3mg/12 hours of CSCIT. The patient noticed a dramatic improvement in her wheeze and chest tightness within 3 days of commencing CSCIT. Patient’s asthma control test improved from 6 to 17. On examination, the patient’s chest was clear. Peak-flow variability significantly decreased to 15% after 1 week of CSIT with increase in her minimum and maximal peak flows. After 2 weeks of CSCIT and self-administration advice, patient was discharged safely on long-term CSCIT.
Discussion
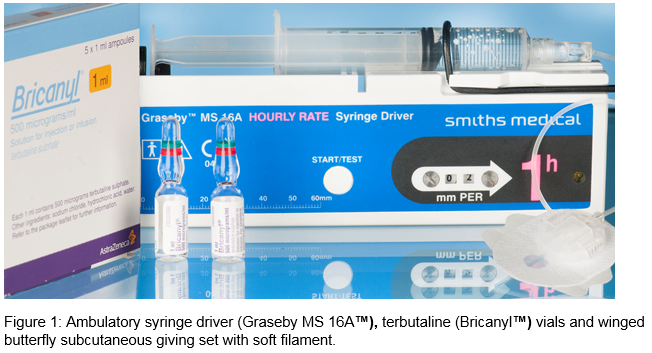
ß-agonists were first developed through modification of the epinephrine molecule to allow selective interaction with the ß-1 and ß-2 receptors rather than the alpha and beta effects of epinephrine.4 Terbutaline was shown to have selective action on ß-2 adrenergic-receptors.5 These receptors are expressed in lung, including airway smooth muscle and inflammatory cells such as mast cells, eosinophils, and lymphocytes. By binding to these receptors it results in G-protein stimulation which increases intracellular cyclic 3’5’-adenosine monophosphate (cAMP) in the bronchus significantly. cAMP then activates protein kinase A that then leads to smooth muscle relaxation and resulting bronchodilation. Its selective ß-2 adrenergic-receptor properties only has a small effect on cAMP concentration in the myocardium thus exerting a lesser effect on heart rate6. CSIT is given using an ambulatory syringe driver (Graseby™ or McKinley™). Terbutaline’s onset of action is 6-15 minutes via the subcutaneous route (approximately 5 minutes when inhaled). CSIT is noted to be more bioavailable than oral dosing. Terbutaline undergoes hepatic metabolism and is renal excreted. Severe renal failure does not lower the total clearance of terbutaline; but more research is warranted to determine if dosing adjustment is warranted in patients with renal failure7. Continuous ß-2 agonist use has been proven to induce in-vivo tachyphylaxis rendering it less effective theoretically with time. This gives reason to reserve it for palliative measures. CSCIT can have adverse effects hence it should only be instituted in experienced centres. Patients have skin reactions like erythema, nodules, rash and infection around the needle site. Other adverse effects include palpitations, hypokalaemia and cramps.
Brittle asthmatics have particularly shown promising responses to CSIT. This difficult phenotype are debilitated with recurring symptoms can also benefit from psychosocial support, allergen avoidance or desensitisation, and dietary advice. The BTS/SIGN guidelines has recognised that CSIT has been reported to be beneficial in severe asthma but warn efficacy has not been assessed in double blind randomised control trials. The side effect profile should ensure this therapy is reserved for patients who have exhausted regular asthma therapy evidenced by GINA and BTS/SIGN guidelines, and in an attempt to palliate symptoms.
Correspondence: A Sahadevan
Department of Respiratory Medicine, AMNCH, Tallaght, Dublin 24
Email: [email protected]
Acknowledgements
J Murphy, Clinical Pharmacist and T Walsh, Medical Photography, Tallaght Hospital
References
1 Mansur AH, Afridi L, Sullivan J, Ayres JG, Wilson D. Continuous terbutaline infusion in severe asthma in adults: a retrospective study of long-term efficacy and safety. J Asthma. 2014;51:1076-82.
2 Jones GH, Scott SJ. Continuous infusions of terbutaline in asthma - a review. J Asthma. 2011;48:753-6.
3 Ayres J. Continuous subcutaneous bronchodilators in brittle asthma. British journal of hospital medicine. 1992;47:569-71.
4 Lemanske R. Beta agonists in asthma: Acute administration and prophylactic use. UpToDate.com [updated April 201511 May 2015].
5 Valentine K. Intravenous terbutaline in severe status asthmaticus https://clinicaltrials.gov/ct2/show/study/NCT00750568?show_desc=Y - desc: US National Institute of Health; 2008 [updated 2008 Sept 8; cited 2015 21 July 2015].
6 Vulliemoz Y, Verosky M, Triner L. Effect of albuterol and terbutaline, synthetic beta adrenergic stimulants, on the cyclic 3',5'-adenosine monophosphate system in smooth muscle. J Pharmacol Exp Ther. 1975;195:549-56.
7 Bastiansen A, Eggert S, Pedersen E. Pharmacokinetics of terbutaline in chronic kidney disease. Eur J Clin Pharmacol. 2013;69:1951-4.
P410