Prevention of Mother-to-Child Transmission in Patients with Chronic Hepatitis B Virus Infection and High Viral Loads
1McCormick CA, 2McCormick PA
1.National Maternity Hospital, Holles St
2.Liver Unit, St Vincent’s University Hospital, Dublin, Ireland.
It is estimated that approximately 248 million people world wide suffer from chronic hepatitis B infection(1). It is a leading cause of chronic liver disease, cirrhosis and hepatocellular carcinoma. In contrast to hepatitis C, where short term drug therapy is curative, current antiviral therapy for hepatitis B suppresses rather than eliminates the virus. Long term antiviral therapy is required to reduce or prevent liver damage. Because of this, prevention of hepatitis B infection is very important. In Western countries viral transmission usually occurs via sexual contact or parenteral routes. In developing countries transmission more commonly occurs around the time of birth or in early childhood. Most countries have now adoped universal hepatitis B vaccination for infants. This has led to a reduction in hepatitis B infection rates and a subsequent reduction in hepatocellular carcinoma rates in children and young adults(2). Hopefully this will translate into a reduction in deaths from cirrhosis and liver cancer in later life.
Unfortunately post natal prophylaxis is not universally effective. High circulating viral loads in the mother, at the time of delivery, are associated with transmission to the infant. Vaccine failure rates increase in parallel with increasing maternal HBV DNA levels (3).
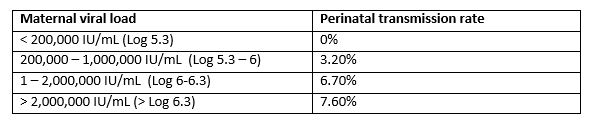 Table 1. Rate of perinatal hepatitis B transmission based on maternal viral load at delivery, in infants receiving post-natal prophylaxis (3).
Table 1. Rate of perinatal hepatitis B transmission based on maternal viral load at delivery, in infants receiving post-natal prophylaxis (3).
In view of this it is recommended that anti-viral therapy be considered in pregnant females with high viral loads (HBV DNA levels > Log 5.3)(4-6). The optimal antiviral treatment in this situation is less clear. In 2016 the American Association for the Study of Liver Disease (AASLD) published a meta-analysis of antiviral therapy to prevent transmission in highly viraemic women(7). Twenty six studies enrolled 3,622 pregnant females. Ten were randomized controlled trials, including a total of 780 participants. The drugs used were lamivudine, telbuvidine and tenofovir. All drugs lowered maternal HBV DNA levels and appeared to reduce rates of maternal to child transmission. However it was noted that most trials were small and there was limited safety data.
In the meantime two relatively large randomized trials using tenofovir to prevent maternal to child transmission in mothers with high viral loads have been reported. Pan et al randomised 200 mothers to receive tenofovir 300mgs daily or placebo starting at 30-32 weeks gestation and continuing till 4 weeks post partum (8). The trial was performed in China. Patients were Hepatitis B e antigen positive mothers with HBV DNA levels greater than 200,000 IU/ml. Mean viral load was Log 8.2 + 0.5 in the Tenofovir group and 8.0 + 0.7 in the placebo group. All infants received immune globulin and vaccine within 12 hours of birth. At time of delivery median maternal HBV DNA levels were Log 4.4 in the tenofovir group compared to log 8 in the placebo group. Viral transmission occurred in 0/92 tenofovir treated pregnancies compared to 6/88 placebo treated (p = 0.01).
The second trial was performed in Thailand and published recently(9). Jourdain and colleagues performed a randomised controlled trial comparing tenofovir to placebo in the third trimester of pregnancy, aiming to prevent perinatal viral transmission(9). Patients were selected for treatment if they were hepatitis B e antigen positive. All infants received hepatitis B immune globulin and vaccination at birth. Treatment was started at 28 weeks gestation and continued till 2 months post partum. Mean hepatitis B DNA viral load was log 7.6 + 1.5 in the tenofovir group and 7.3 + 1.7 in the placebo group. Three hundred and thirty-three women were enrolled and 294 were included in the main analysis. Mean hepatitis B viral load at the time of delivery was 7.3 + 1.7 in the placebo group and 4.0 + 1.6 in the tenofovir group (p< 0.001). Hepatitis B transmission to the infant was documented in no subjects (0/147) receiving tenofovir and in 3/147 in the placebo group. The difference did not reach statistical significance. No significant safety issues were identified during the trial.
Taken together these two very similar trials suggest that tenofovir is effective in preventing maternal to child transmission in mothers with high hepatitis B viral loads. Combining the studies, viral transmission occurred in 9/235 patients receiving placebo compared to 0/239 receiving tenofovir (p < 0.01). It is a little surprising that there was such a difference in transmission rates between the placebo groups in the two trials. 2% versus 6.8%. The reasons for this are not clear. In the Thai trial the median time to administration of vaccine and immune globulin was 1.2 hours after birth in the placebo group. In the earlier Chinese trial it is stated that they were administered within 12 hours of birth. It is certainly plausible that earlier administration of immune globulin may improve efficacy.
These two trials provide strong evidence that tenofovir treatment in the third trimester reduces mother to child transmission rates in patients with high viral loads and should now be considered standard of care. The low transmission rates in the placebo group of the Thai trial would suggest that immune globulin and vaccine should be administered as soon after birth as possible when mothers have chronic hepatitis B virus infection.
Corresponding Author
McCormick PA,
National Maternity Hospital,
Holles St and *Liver Unit,
St Vincent’s University Hospital,
Dublin,
Ireland.
Email: [email protected]
References
1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546-1555. 2. Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, et al. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology 2016;151:472-480 e471. 3. Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat 2012;19:e18-25. 4. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-398. 5. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98. 6. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-1599. 7. Brown RS, Jr., McMahon BJ, Lok AS, Wong JB, Ahmed AT, Mouchli MA, Wang Z, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology 2016;63:319-333. 8. Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, Zhang H, et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N Engl J Med 2016;374:2324-2334. 9. Jourdain G, Ngo-Giang-Huong N, Harrison L, Decker L, Khamduang W, Tierney C, Salvadori N, et al. Tenofovir versus Placebo to Prevent Perinatal Transmission of Hepatitis B. N Engl J Med 2018;378:911-923.
P831
