Rotavirus Gastroenteritis is Associated with Increased Morbidity and Mortality in Children with Inherited Metabolic Disorders
A Smith1, M Mannion1, P O’Reilly1, E Crushell1, J Hughes1, I Knerr1, P Gavin2, A Monavari1
1National Centre for Inherited Metabolic Disease, Temple Street Children’s University Hospital, Dublin
2Department of Infectious Disease, Temple Street Children’s Hospital University Hospital, Dublin
Abstract
Rotavirus is the leading cause of infantile diarrhoea worldwide in children <5 years1. Although mortality rates are low in Ireland, certain populations are more susceptible to the associated morbidity and mortality of infection. A retrospective chart review of 14 patients with confirmed IMDs who were admitted to Temple Street Children’s Hospital between 2010 to 2015 with rotavirus infection were compared with 14 randomly selected age matched controls. The median length of stay was 7 days (SD25.3) in IMD patients versus 1.5 days (SD 2.1) in the controls. IV fluids were required on average for 4.5 days (range 0-17) in IMD patients versus 0.63 days (range 0-3) in controls. This report highlights the increased morbidity of rotavirus infection in patients with IMD compared to healthy children. This vulnerable population are likely to benefit from the recent introduction of the rotavirus oral vaccination in October 2016.
Introduction
Rotavirus infection is the leading cause of severe gastroenteritis in the paediatric population globally 2. In Ireland, rotavirus infection became a notifiable disease in 2004 and according the Health Protection Surveillance Centre report on the Epidemiology of Rotavirus in Ireland during 2013 there were 2,514 cases with a crude incidence of 54.8/100,000 population 3. Virtually all children by the age of six years have experienced at least one episode of rotavirus gastroenteritis and 66.6% (n=1,674) of Irish cases in 2013 were aged two years and under 3.
Rotavirus infection is seasonal and transmission is typically via the faecal-oral route. Symptoms usually include vomiting, watery diarrhoea and pyrexia which may necessitate hospitalisation secondary to dehydration. A recent UK study demonstrated that in 52% of families with one child infected with rotavirus acute gastroenteritis at least one other family member developed a secondary case of gastroenteritis. Additionally, 69% of working parents missed work while caring for a child with rotavirus infection 4. Rotavirus infection can induce significant morbidity in at risk populations such as the elderly, infants, immunocompromised, those with chronic disease and institutionalised patients. One such vulnerable population is those with inherited metabolic disease. Due to their innately abnormal energy metabolism those with IMD are exquisitely vulnerable to dehydration, hypoglycaemia and acid-base disturbances which rotavirus infection may rapidly induce.
The aim of this study was to assess the morbidity and mortality associated with rotavirus gastroenteritis infection in a cohort of paediatric patients with IMD compared to age matched controls.
Methods
Fourteen children who attend the National Centre for Inherited Metabolic Diseases who were admitted with rotavirus gastroenteritis from 2010-2015 were identified in the microbiology laboratory database. These fourteen children with IMD were compared to fourteen otherwise healthy children of the same age, +/- 8 months, admitted with rotavirus gastroenteritis. A retrospective chart review was conducted to determine: age; reason for admission; co-morbid conditions; length of stay; length of intravenous fluid treatment, requirement for intravenous lipids; central venous access; number of blood draws and outcome of rotavirus infected patients with IMD compared to controls. Ethical approval was obtained from the Research Department at CUH.
Results
From 2010-15, 14 patients with IMD and 14 healthy matched controls with rotavirus gastroenteritis were identified. The metabolic disorders included homocystinuria (n=1), I Cell Disease (n=1), Mucopolysaccharidosis 1 (Hurler Syndrome) (n=1), Non-ketotic hyperglycinaemia (NKH) (n=1), Alpha-mannosidosis (n=1), Methylmalonic acidemia (n=2), Glutaric aciduria type 1 (n=1), Complex 1 Mitochondrial Disorder (n=1), Alper’s Syndrome (n=1), recurrent ketotic hypoglycaemia (n=1), Leigh disease (n=1), Ornithine transcarbamylase deficiency (n=1) and Kearns Sayre syndrome (n=1). Clinical presentations among the IMD cohort included apnoea (n=1), pyrexia (n=1), vomiting and diarrhoea (n=5), hypoglycaemia (n=2), pancreatitis (n=1) and decreased level of consciousness (n=1). Three IMD patients admitted electively contracted rotavirus infection in hospital. In the control population, all patients had symptoms of gastroenteritis with vomiting and diarrhoea.
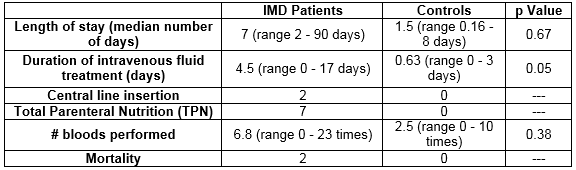
Median length of stay was 7 days (range 2-90) in IMD patients with rotavirus infection versus 1.5 days (range 0.16-7) in the healthy controls with rotavirus infection, p=0.67. Average duration of intravenous fluid treatment was 4.5 days (range 0-17) versus 0.63 days (range 0-3) in the control group, p=0.05. 2 patients with IMD patients required central line insertion and 7 patients with IMD required Total Parenteral Nutrition (TPN), no patient in the control group required central line insertion or TPN. Bloods were performed on average 6.8 times (range 0-23) in the metabolic group versus 2.5 times (range 0-10) in the controls, p=0.38. 2/14 patients (14%) in the metabolic group died during their admission. Their underlying metabolic conditions were Leigh’s Disease and I Cell Disease which are life limiting conditions. They were confirmed with rotavirus infection on day 38 and day 8 of admission to hospital respectively, which resulted in acute decompensation, however it was not a direct cause of mortality in both cases.
Discussion
Prior to the implementation of rotavirus vaccination in Europe in 2006 it was estimated that rotavirus infection caused 231 deaths and over 87,000 hospitalisations in Europe every year 5. The World Health Organisation recommended the introduction of rotavirus vaccination to national immunisation programmes in 2009 and by the start of 2014 rotavirus vaccination had commenced nationally in Austria, Belgium, Luxembourg, Finland, Greece, Norway and the UK 5, 6.
In Ireland all children born on or after 1 October 2016 will receive the rotavirus oral vaccine at 2 and 4 months of age. This vaccine provides 95% protection against severe rotavirus disease 7. It is estimated that rotavirus vaccination will prevent 2,038 GP visits, 3,271 emergency department attendances and 2,499 hospital admissions annually in Ireland 8. The oral rotavirus vaccine was introduced in England and Wales in July 2013 and a study by Atchison et al in 2015 has demonstrated a 77% reduction in laboratory confirmed rotavirus infection and a 26% decline in all cause acute gastroenteritis hospitalisations in 2013/2014 compared to pre vaccination years 9. A 2015 US report documented that following introduction of rotavirus vaccination in 2006, ‘all cause acute gastroenteritis hospitalisation rates among US children younger than 5 years declined by 31% - 55% in each of the postvaccine years from 2008 through 2012’ 10. Such findings are also echoed in Spanish and Australian populations 11, 12. Additionally, in a cohort of 250, 601 children, Payne et al report an 18-21% reduction in the risk of seizure requiring hospitalisation or emergency department care in the year following vaccination for children who received a full course of rotavirus vaccination compared with unvaccinated children 13.
Our report demonstrates that children with IMD who contract rotavirus have longer hospital admissions, require a longer duration of IV fluids, are more likely to require total parenteral nutrition and more frequent blood sampling than healthy children with rotavirus infection. Limitations of this study are that it was retrospective in nature and the sample size was small.
In conclusion, children with IMDs are particularly vulnerable to rotavirus gastroenteritis as it can induce an acute decompensation, hypoglycaemia, liver dysfunction and even shock. Intensive fluid and nutritional management in specialised centres is vital during such episodes. The introduction of the rotavirus vaccine should offer additional protection to this at risk patient population however further studies will be required to assess this potential effect.
Correspondence: Dr. Aisling Smith, National Centre for Inherited Metabolic Disease, Temple Street Children’s University Hospital, Dublin.
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
1. Rotavirus surveillance --- worldwide, 2009. MMWR Morbidity and mortality weekly report. 2011;60(16):514-6.
2. Aliabadi N, Tate JE, Haynes AK, Parashar UD. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination-United States, 2000-2014. MMWR Morbidity and mortality weekly report. 2015;64(13):337-42.
3. Health Protection Surrveillance Centre H. Epidemiology of Rotavirus in Ireland. 2013.
4. Marlow R, Finn A, Trotter C. Quality of life impacts from rotavirus gastroenteritis on children and their families in the UK. Vaccine. 2015;33(39):5212-6.
5. Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006-2014. Vaccine. 2015;33(18):2097-107.
6. Rotavirus vaccines. WHO position paper - January 2013. Releve epidemiologique hebdomadaire. 2013;88(5):49-64.
7. WHO Global rotavirus surveillance network - a strategic review of the first 5 years (2008-2012). Releve epidemiologique hebdomadaire. 2014;89(30):340-4.
8. Pharmacoeconomics NCf. Economic Evaluation of Universal Rotavirus Vaccination of Infants in Ireland. 2010.
9. Atchison CJ, Stowe J, Andrews N, Collins S, Allen DJ, Nawaz S, Brown D, Ramsay ME, Ladhani SN. Rapid Declines in Age Group-Specific Rotavirus Infection and Acute Gastroenteritis Among Vaccinated and Unvaccinated Individuals Within 1 Year of Rotavirus Vaccine Introduction in England and Wales. The Journal of infectious diseases. 2016;213(2):243-9.
10. Leshem E, Tate JE, Steiner CA, Curns AT, Lopman BA, Parashar UD. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. Jama. 2015;313(22):2282-4.
11. Redondo O, Cano R, Simon L. Decline in rotavirus hospitalizations following the first three years of vaccination in Castile-La Mancha, Spain. Human vaccines & immunotherapeutics. 2015;11(3):769-75.
12. Davey HM, Muscatello DJ, Wood JG, Snelling TL, Ferson MJ, Macartney KK. Impact of high coverage of monovalent human rotavirus vaccine on Emergency Department presentations for rotavirus gastroenteritis. Vaccine. 2015;33(14):1726-30.
13. Payne DC, Baggs J, Zerr DM, Klein NP, Yih K, Glanz J, Curns AT, Weintraub E, Parashar UD. Protective association between rotavirus vaccination and childhood seizures in the year following vaccination in US children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(2):173-7.
(P546)