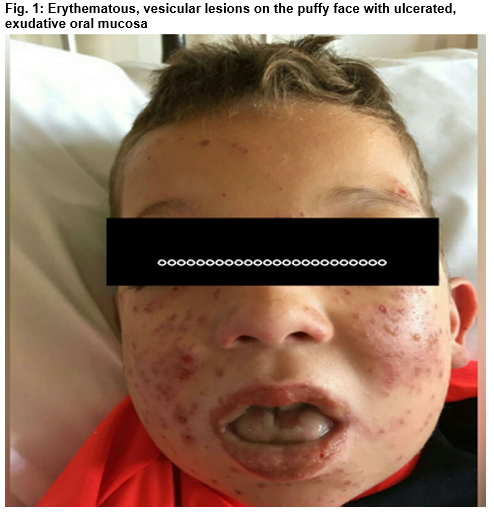
Stevens - Johnson Syndrome Induced by Combination of Lamotrigine and Valproic Acid in a 9-Year-Old Boy
K Maduemem, A Vatca, T O’Neill, D Buckley
Department of Paediatrics, Cork University Hospital, Wilton, Cork.
Abstract
We describe the case history of a 9-year-old boy who developed Stevens-Johnson syndrome (SJS) following concomitant use of valproic acid and lamotrigine. He presented with rash and fever several weeks after introduction of lamotrigine, having been on valproic acid for seizure disorder. SJS happens to be one of the rare adverse reactions of antiepilepsy drugs (AED). Management is mainly supportive with care escalation when necessary because of the significant morbidity.
Introduction
Steven-Johnson syndrome is an immune complex mediated hypersensitivity reaction that characteristically involves skin and mucous membranes. Antiepileptic drugs are strongly associated with SJS. Combined usage of AED may cause potential pharmacokinetic and pharmacodynamic interactions which may result in more adverse effects than might occur when the AED is taken singly. We report a case of SJS likely induced by the use of lamotrigine and valproic acid regimen.
Case Report
A 9-year-old boy presented with 3 day history of high grade fever and generalised rash. He is a known seizure disorder patient for the preceding 2 years with background of XYY karyotype, and attention deficit hyperactivity disorder on valproic acid (Epilim) He had the first episode of generalised tonic clonic seizure two months before index admission. Lamotrigine (Lamictal) was introduced at a dose of 25mg nocte alternate days (0.4mg/kg/day). He developed symptoms 6 weeks into the therapy in addition to Epilim 400mg BID. On physical examination, he was very distressed and febrile. Dermatological examination revealed vesicular rash on the puffy face with haemorrhagic crust, vesicles on the torso, upper back, arms. There was minimal scrotal vesicular rash. Eye exam revealed chemosis, significant membrane formations on the palpebral conjunctiva bilaterally. Oral cavity showed swollen tongue with multiple ulcers and some bleeding spots.
Investigations included CRP 81. Haemoglobin level (12.9), white cell count (15.3), platelet count (155). Renal and liver profile remained normal. HSV1 and 2 DNA: not detected, CMV IgM, EBV IgM, Mycoplasma IgM: negative. ASOT <200. Negative urine culture, blood culture and eye swab. He was diagnosed with SJS secondary to possibly Lamictal viral infection. Lamictal was withdrawn immediately on admission.
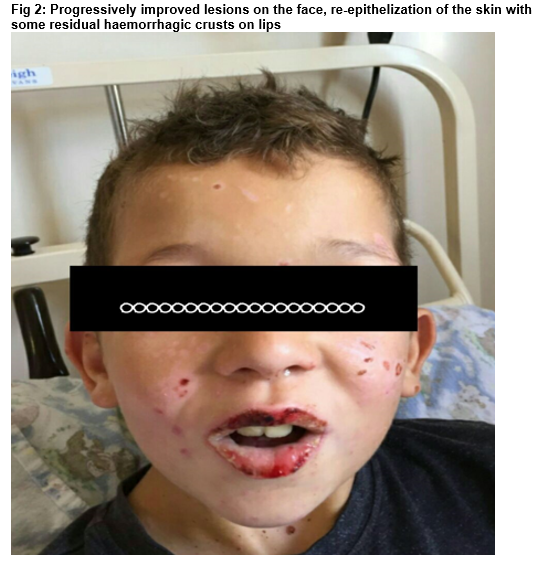
He was commenced on intravenous aciclovir (250mg/bsa) and ceftriaxone (80mg/kg/day), oral azithromycin, Intravenous fluid. He was on topical antibiotics and steroids for the eyes, mouth wash and fucidin H ointment. He required aggressive analgesia including intravenous morphine (PCA: 0.3mg). His clinical state steadily improved over the following days. He made an excellent recovery and was discharged after twelve days on admission. He has been seen in clinic 4 months post discharge and still off AED.
Discussion
SJS is an immune-complex mediated hypersensitivity reaction that characteristically involves skin and mucous membrane. It is rare but considered a dermatological emergency, as it is potentially fatal. The incidence of SJS is estimated to be between 1.1 and 7.7 cases per million, with about 16% of cases showing a past history of short term use of AED1.
Our patient was a 9-year-old epileptic boy previously on valproic acid and was subsequently introduced in lamotrigine before the new onset rash developed. The initial diagnosis of SJS was sustained, although we had differentials as the likely triggers. Hypersensitivity reactions can occur with almost all AED with cutaneous side effects occurring in 3 to 10% patients, and rash usually developing in the first few weeks in a small number of patients2. When seizures are poorly controlled, AED are used in combination, leading to potential pharmacokinetic and pharmacodynamic interactions that cause more adverse effects than might occur in monotherapy3.
It has been suggested in the literature that rapid elevation of lamotrigine levels may increase the occurrence of lamotrigine-related skin lesions and rash4. Valproic acid, a glucuronide inhibitor, decreases lamotrigine clearance by 60%; the combined usage of these drugs may easily result in increased lamotrigine levels. Lower starting dose of lamotrigine and slower dose escalations are recommended in patients taking valproate; starting dose of 0.15mg/kg/day5. In a major retrospective study of 1,890 outpatients with epilepsy, researchers assessed the rates of rash associated with 15 AEDs. The highest rash rates occurred with phenytoin (5.9%), lamotrigine (4.8%) and carbamazepine (3.7%)6.
Treatment of SJS is primarily supportive which includes strict isolation, increasing caloric intake, preventing superinfection and sepsis, and correcting electrolyte disturbance7. The role of systemic corticosteroids is controversial, although have been the main stay of treatment.
Correspondence:
Kene Maduemem, Department of Paediatrics, Cork University Hospital, Wilton, Cork.
Email: [email protected]
Conflict of Interest
None declared.
References
1. Jao T, Tsai TH, Jeng JS. Aggrenox (Asasantin retard)-induced Stevens-Johnsons syndrome. Br J Clin Pharmacol. 2009;67:264-5
2. Mockenhaupt M, Messenheimer J, Tennis P, Schlingmann J. Risk of Stevens-Johnsons syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology. 2005;64(7):1134-1138.
3. Yalcin B, Karaduman A: Stevens-Johnsons syndrome associated with concomitant use of lamotrigine and valproic acid. J Am Acad Dermatol, 43: 703-6, 2012.
4. Cramer JA, Mintzer S, Wheless J: Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother, 10:885-91, 2010.
5. https://www.drugs.com>dosage>lamictal
6. Arif H, Buchsbaum R, Weintraub D.: Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology, 68: 1701-9, 2007.
7. Mockenhaupt M. The current understanding of Stevens-Johnsons syndrome and toxic epidermal necrolysis. Curr Allergy Clin Immunol. 2007;20:124-8.
p586