The Management of Incidental Prostate Cancer Following TURP
D.M. Matanhelia1, S. Croghan1, G.J. Nason2, C. O’Connell2, D.J. Galvin1,2
1. Department of Urology, St Vincent’s University Hospital, Dublin 4
2. Department of Urology, Mater Misericordiae University Hospital, Dublin 7
Abstract
Aims
The aim of this study was to assess the incidence, management and outcomes of incidentally diagnosed prostate cancer following TURP.
Methods
A retrospective review was performed using the histopathological departments’ database of all patients who underwent a TURP across two university teaching hospitals over a ten year period.
Results
During the study period, a total of 826 patients underwent a TURP. 72 (10.3%) had an incidental diagnosis of CaP following TURP. 46 (63.9%) were managed expectantly while 26 (36.1%) underwent active treatment. Overall mortality was 29.2% (n=21) while cancer specific mortality was 6.9% (n=5). All these patients were in the hormonal treatment sub-group.
Conclusion
Our study demonstrates an expectant approach is favourable in low risk disease. Curative treatment does need to be considered for younger patients with a long life expectancy or patients with higher risk disease.
Introduction
Prostate cancer (CaP) is the most common non-cutaneous cancer diagnosed in Ireland with a cumulative lifetime risk of 1 in 7 men1. At autopsy, up to 60% of 80 years olds have latent CaP2 while up to 80% have benign prostatic hyperplasia (BPH) 3. The detection of CaP incidentally has fallen in the post prostate specific antigen (PSA) era from ~ 20% to ~5%4. Incidental CaP detected at transurethral resection of prostate (TURP) can be sub-classified into pT1a (<5% of prostate chips) and pT1b (>5% of prostate chips) and the management subsequently stratified.
There is no clear consensus regarding the management of incidentally diagnosed CaP at TURP. Treatment approaches reported include watchful waiting (WW), deferred treatment and curative treatment5,6. Data from the PIVOT trial demonstrated no improvement in mortality in men with early localised CaP treated with radical prostatectomy compared to watchful waiting7. However, Magheli et al, demonstrated no difference in recurrences rates following radical treatment of pT1a and pT1b CaP8.Capitanio et al, demonstrated high 5 and 10 year biochemical free survival rates for a cohort of patients who underwent radical prostatectomy for T1a/b CaP6, however only 15% of patients required curative treatment in a series described by Ahmad5.
Furthermore, there is lack of uniformity regarding the pathological handling of TURP specimens. For a standard TURP, indicated for BPH, only a portion of the whole specimen is analysed. Perera et al, advocate analysis of the whole specimen in younger men (<65 years old) as this may change the long term management of the incidental CaP in a younger fit patient9.
The aim of this study was to assess the incidence, management and outcomes of incidentally diagnosed CaP following TURP.
Methods
A retrospective review was performed using the histopathological departments’ database of all patients who underwent a TURP across two university teaching hospitals over a ten year period between 2007 and 2016. A chart review was performed of all cases.
Patients with a prior histological proven diagnosis of CaP were excluded. All other cases were included in our analysis. The management of patients with incidental prostate cancer was categorised in active (surgery, radiotherapy or hormonal treatment) or expectant (watchful waiting) treatment.
The number of months of survival was calculated as the number of months from date of surgery until December 2017. Deaths were classified as CaP related or non cancer related.
TURP specimens were analysed by a standard histopathological protocol in each hospital10. For specimens weighing ≤ 10 g, the entire specimen was processed and examined histologically. For specimens weighing > 10 g, the first 10 g were processed with an additional 2 g for every 10 g of tissue resected. The specimens were fixed in 10% neutral buffered formalin with overnight processing. A single hematoxylin and eosin-stained section was cut from each block and examined histologically. All foci were outlined on the glass slides and an estimate of tumor volume as a visual estimate of the percentage of surface area of tumor to the entire specimen determined. Gleason scoring was based on the 2005 International Society of Urological Pathology (ISUP) consensus guidelines.
Reporting of incidental cancer on TURP aligned with the CaP recommendations. pT1a disease was defined as incidental tumor in ≤ 5% of TURP specimens. pT1b disease was defined as incidental tumor in > 5% of TURP specimens.
Results
During the study period, a total of 826 patients underwent a TURP. 127 (15.4%) patients had a prior history of CaP and were excluded from further analysis.
Of the remaining 699 patients, 72 (10.3%) had an incidental diagnosis of CaP following TURP. 37 (51.4%) had pT1a (<5% of prostate chips) disease while 35 (48.6%) had pT1b (>5% of prostate chips) disease. Patient and pathological demographics are recorded in Table 1.
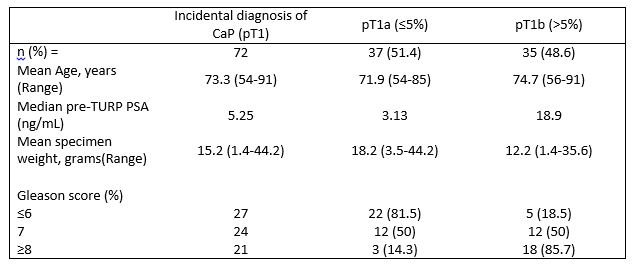
Table 1. Patient demographics and pathological parameters
Of the 72 patients with an incidental diagnosis of prostate cancer, 46 (63.9%) were managed expectantly while 26 (36.1%) underwent active treatment (Table 2). 7 (9.7%) patients underwent radical curative treatment; 1 (1.4%) robotic assisted radical prostatectomy and 6 (8.3%) radical external beam radiotherapy. 19 (26.4%) patients were commenced on hormonal treatment.
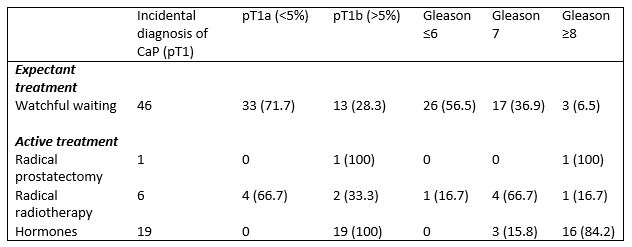
Table 2. Treatment according to T stage and Gleason score
Table 3 shows pathological characteristics and survival among the different treatment groups. Overall mortality was 29.2% (n=21) while cancer specific mortality was 6.9% (n=5).
A total of 46 patients were managed expectantly with WW. Mean overall survival in this group was 67.2 months for the pT1a group and 46.5 months for the pT1b group. There were no cancer-specific deaths in this cohort, 13 patients died of other causes.
Seven patients were given curative treatment. Six patients had radiotherapy; four with pT1a and two with pT1b disease. Mean age was 63.3 years and 69 years in the pT1a and pT1b group respectively. Median pre-TURP PSA was 7.2 in the pT1a group with a mean Gleason grade of 7.3, compared to a PSA of 19.1 and mean Gleason grade of 7 in the pT1b group. All patients were alive at follow-up, with a mean overall survival of 68.8 months, and no patient had a biochemical recurrence. One patient had a robotic radical prostatectomy. This patient was 61 years with a pre-TURP PSA of 47, pT1b Gleason 9 disease, and no radiological evidence of metastatic disease. This patient was alive with an undetectable PSA at 85 months. Nineteen patients were given hormonal treatment with an LHRH analogue. Mean age was 76.3 years, and median pre-TURP PSA was 25. All patients had pT1b disease, and mean Gleason grade of 8.7. Seven patients had metastatic disease at the time of diagnosis. Mean overall survival was 50.3 months. Five patients died from metastatic prostate cancer at a mean of 53.8 months, and 3 from other causes.
In the whole cohort, cancer-specific mortality was 6.9% (n=5). All these patients were in the hormonal treatment sub-group. Mean age at diagnosis was 76.6 years (range 72-83 years), and mean age at death was 81.2 years (range 76-86 years). Mean pre-TURP PSA was 33.7, and all patients had pT1b Gleason 9 disease. Two patients had metastatic disease at the time of diagnosis. Mean time to progression of disease was 37.6 months, and mean overall survival in this group was 53.8 months.
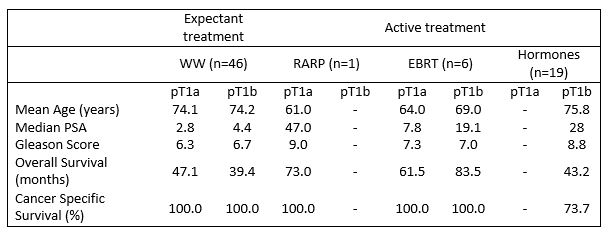
Table 3. Pathological characteristics and survival by treatment group
Discussion
CaP is diagnosed incidentally in ~10% of TURP specimens in our study. There is a lack of consensus regarding the management of these patients. Expectant management shows favourable outcomes in patients with low risk disease. Only 10% in our cohort underwent curative treatment, with a 100% biochemical recurrence free survival rate. There were 5 cancer related deaths - all had high risk disease (high PSA and Gleason 9 disease).
Ahmad et al, reported similar outcomes to our study - overall mortality of 27% and cancer specific mortality of 11.6% (compared to 29% and 6.9%) despite actively treating a higher proportion (49% vs 36%). They did however note a higher proportion of pT1b disease (70.1% vs 48.6%)5. Both these studies highlight the role of expectant treatment.
Contrary to our approach, some studies question the need for radical treatment of pT1 disease. Capitanio et al, performed a radical prostatectomy on 126 patients with pT1 disease. Not surprisingly they describe high biochemical free survival rates. However, 5% of patients harboured T3 disease. They noted PSA following TURP and Gleason score of the specimen as the only predictors of recurrence following radical treatment6. The ability to predict disease progression, residual disease and identification of high risk patients is key. The risk of progression of untreated pT1a CaP after 5 years is only 5%, but can increase to 50% after 10–13 years. In contrast most patients with pT1b tumours progress and show lower cancer specific survival after 5 years11. The debate centres on the argument of overtreatment of low risk prostate cancer. The PIVOT trial demonstrated no survival benefit for radical prostatectomy in early localised disease7. Active surveillance has been advocated as the solution to the over-diagnosis and over-treatment of low risk disease12.
The challenge regarding incidental CaP is identifying those at risk of higher risk disease. Magheli et al, questioned the sub-classification of pT1 disease. In that series, biochemical recurrence-free survival rates were significantly better in clinical stage T1a cases than in T1b and T1c cases. However, T1 substaging failed to reach independent predictor status for biochemical recurrence after accounting for race, Gleason score at surgery for BPH, and PSA8.
With the advent of newer technology such as laser prostatectomy, there is a lack of specimen for histological analysis. Meeks et al, demonstrated that with the use of PSA the number of clinically significant CaP diagnosed is low (0.26%)13. In view of this, it is hard to recommend a radical prostatectomy for T1 disease.
The diagnostic pathway for CaP has changed in recent years. The PROMIS trial has highlighted the diagnostic accuracy of MRI for the detection of high grade prostate cancer14. MRI can accurately predict pathological markers such as extracapsular extension and seminal vesicle invasion which impact on treatment choices15. Furthermore, targeted biopsy (trans-rectal or trans-perineal) of MRI lesions accurately correlates with pathological outcomes giving the patient a clearer picture in advance of a treatment decision16. The PRECISION trial demonstrated the use of risk assessment with MRI before biopsy and MRI-targeted biopsy was superior to standard transrectal ultrasonography-guided biopsy in biopsy naive men17. Ultimately, patients will now undergo further investigation to stage their disease prior to any radical treatment following the incidental diagnosis of CaP. Ideally, a risk nomogram incorporating PSA, TURP pathology and MR findings needs to be developed.
A limitation of our study is that only patients with a prior histological diagnosis of prostate cancer were excluded. Some patients with a high PSA or malignant gland on examination were likely to have prostate cancer and were included. Due to the retrospective nature of the study, the treatment options were not study protocol driven, and management was decided between the treating urologist and the patient. Furthermore, the pathological analysis was not centralised, and each specimen was reported individually at the treating institution.
Debate remains regarding the management of incidental prostate cancer at TURP. Our study demonstrates an expectant approach is favourable in low risk disease. Curative treatment does need to be considered for younger patients with a long life expectancy or patients with higher risk disease.
Conflict of Interest
There are no conflicts of interest to disclose.
Corresponding Author
Mr Gregory Nason,
FRCS Urol. Specialist Registrar in Urology,
Department of Urology,
Mater Misericordiae University Hospital,
Dublin 7
Email: [email protected]
References
1. https://www.ncri.ie/sites/ncri/files/factsheets/prostate.pdf
2. Zlotta AR, Egawa S, Pushkar D, Govorov A, Kimura T, Kido M, Takahashi H, Kuk C, Kovylina M, Aldaoud N, Fleshner N, Finelli A, Klotz L, Sykes J, Lockwood G, van der Kwast TH. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. J Natl Cancer Inst. 2013 Jul 17;105(14):1050-8.
3. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984 Sep;132(3):474-9.
4. Jones JS, Follis HW, Johnson JR. Probability of finding T1a and T1b (incidental) prostate cancer during TURP has decreased in the PSA era. Prostate Cancer Prostatic Dis. 2009;12(1):57-60
5. Ahmad S, O'Kelly F, Manecksha RP, Cullen IM, Flynn RJ, McDermott TE, Grainger R, Thornhill JA. Survival after incidental prostate cancer diagnosis at transurethral resection of prostate: 10-yearoutcomes. Ir J Med Sci. 2012 Mar;181(1):27-31.
6. Capitanio U, Scattoni V, Freschi M, Briganti A, Salonia A, Gallina A, Colombo R, Karakiewicz PI, Rigatti P, Montorsi F. Radical prostatectomy for incidental (stage T1a-T1b) prostate cancer: analysis of predictors for residual disease and biochemical recurrence. Eur Urol. 2008 Jul;54(1):118-25.
7. Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, Gingrich JR, Wei JT, Gilhooly P, Grob BM, Nsouli I, Iyer P, Cartagena R, Snider G, Roehrborn C, Sharifi R, Blank W, Pandya P, Andriole GL, Culkin D, Wheeler T; Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012 Jul 19;367(3):203-13.
8. Magheli A, Rais-Bahrami S, Carter HB, Peck HJ, Epstein JI, Gonzalgo ML. Subclassification of clinical stage T1 prostate cancer: impact on biochemical recurrence following radical prostatectomy. J Urol. 2007 Oct;178(4 Pt 1):1277-80
9. Perera M, Lawrentschuk N, Perera N, Bolton D, Clouston D. Incidental prostate cancer in transurethral resection of prostate specimens in men aged up to 65 years. Prostate Int. 2016 Mar;4(1):11-4.
10. Srigley JR, Humphrey PA, Amin MB, Chang SS, Egevad L, Epstein JI, Grignon DJ, McKiernan JM, Montironi R, Renshaw AA, Reuter VE, Wheeler TM; Members of the Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with carcinoma of the prostate gland. Arch Pathol Lab Med. 2009 Oct;133(10):1568-76.
11. Helfand BT, Mongiu AK, Kan D, Kim DY, Loeb S, Roehl KA, Meeks JJ, Smith ND, Catalona WJ. Outcomes of radical prostatectomy for patients with clinical stage T1a and T1b disease. BJU Int. 2009 Aug;104(3):304-9.
12. Goldberg H, Klaassen Z, Chandrasekar T, Fleshner N. Preventing clinical progression and need for treatment in patients on active surveillance for prostate cancer. Curr Opin Urol. 2018 Jan;28(1):46-54
13. Meeks JJ, Maschino AC, McVary KT, Sandhu JS. Clinically significant prostate cancer is rarely missed by ablative procedures of the prostate in men with prostate specific antigen less than 4 ng/ml. J Urol. 2013 Jan;189(1):111-5
14. Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP, Oldroyd R, Parker C, Emberton M; PROMIS study group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017 Feb 25;389(10071):815-822.
15. Somford DM, Hamoen EH, Fütterer JJ, van Basten JP, Hulsbergen-van de Kaa CA, Vreuls W, van Oort IM, Vergunst H, Kiemeney LA, Barentsz JO, Witjes JA. The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low, intermediate and high risk prostate cancer. J Urol. 2013 Nov;190(5):1728-34.
16. Lee SM, Liyanage SH, Wulaningsih W, Wolfe K, Carr T, Younis C, Van Hemelrijck M, Popert R, Acher P. Toward an MRI-based nomogram for the prediction of transperineal prostate biopsy outcome: A physician and patient decision tool. Urol Oncol. 2017 Nov;35(11):664.e11-664.e18.
17. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, Briganti A, Budäus L, Hellawell G, Hindley RG, Roobol MJ, Eggener S, Ghei M, Villers A, Bladou F, Villeirs GM, Virdi J, Boxler S, Robert G, Singh PB, Venderink W, Hadaschik BA, Ruffion A, Hu JC, Margolis D, Crouzet S, Klotz L, Taneja SS, Pinto P, Gill I, Allen C, Giganti F, Freeman A, Morris S, Punwani S, Williams NR, Brew-Graves C, Deeks J, Takwoingi Y, Emberton M, Moore CM; PRECISION Study Group Collaborators. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018 May 10;378(19):1767-1777
P866
