The Prevalence of Pseudomonas Aeruginosa Infection Over a Ten-Year Period in Children with Cystic Fibrosis
K. Al Shidhani1, 2, R. O’Reilly2, S. Javadpour2, N. O’Sullivan3, P. McNally1, 2, 4, D.W. Cox1, 2, 5
1. National Children’s Research Centre, Our Lady’s Children Hospital, Crumlin, Dublin, Ireland
2. Department of Respiratory Medicine, Our Lady’s Children Hospital, Crumlin, Dublin, Ireland
3. Department of Microbiology, Our Lady’s Children Hospital, Crumlin, Dublin, Ireland
4. Department of Pediatrics, Royal College of surgeons in Ireland, Dublin, Ireland
5. School of Medicine, University College Dublin, Dublin, Ireland
Abstract
Background
Pseudomonas aeruginosa (PA) infection is associated with an increased morbidity and adverse prognosis in children with Cystic Fibrosis(CF). The aim of the study was to evaluate the prevalence and characteristics of PA over a ten year period at a single paediatric tertiary referral centre in Ireland.
Methods
This was a retrospective cross-sectional study. Patient’s case notes, microbiology laboratory results and CF Registry of Ireland(CFRI) data were used to collect the data.
Results
The overall chronic PA infection prevalence was 28.1%(45/160) in 2004 and 21.3%(35/164) in 2014. In 2004, 54/160(33.8%) patients were never infected with PA, 27/160(16.9%) were free for 12 months and 34/160(21.3%) were intermittently infected. In 2014; 80/164(49%) patients, 38/164(23.2%) and 11/164(6.7%) were never infected, free for 12 months and intermittently infected respectively.
Conclusion
There has been a decline in the overall prevalence of PA infection and a change in the pattern of prevalence over the last decade at our Centre.
Introduction
Cystic fibrosis (CF) is a life-limiting disease with pulmonary insufficiency as the main cause of mortality1. Pseudomonas aeruginosa (PA) is the major pathogen involved in the decline of lung function in children with CF2,3,4,5. It is well known that chronic infection with PA is associated with an increased morbidity and adverse prognosis in children with CF6,7,8,9. Chronic PA infection, especially the mucoid phenotype growth, can rarely be eradicated10 However, a newly acquired PA infection can often be eradicated successfully from the lower airway10,11,12 with success rates above 75%13,21. Successful PA infection eradication has been associated with slower disease progression in CF11,12,13.
In 2015, the US Cystic Fibrosis Foundation Registry reported that 47 % of CF patients had positive PA infection (17% intermittent infection and 30 % chronic infection based on Leed’s criteria) with a median age of 5.5 years14. They have reported that around 30.4 % of CF patients younger than 18 year of age had positive PA culture in 2015. From an Irish perspective, a recent study demonstrated an overall prevalence of PA infection of 31% (19% chronic and 12% intermittent infection). This study included all patients (children above 4 years of age and adults) with CF registered in the Cystic Fibrosis Registry of Ireland (CFRI) database in 201316. To date, there has been no study examining the change in prevalence of PA infection over time in purely paediatric CF population in Ireland. Our aim was to estimate the change in prevalence of PA infection at our centre over the period of a decade.
Methods
A retrospective observational study was performed at Our Lady’s Children’s Hospital, Crumlin (OLCHC), Dublin, the largest paediatric tertiary CF referral centre in Ireland. Patient’s case notes, microbiology laboratory results and data from the Cystic Fibrosis Registry of Ireland (CFRI) database were used as sources for the data collection. We compared the prevalence of PA infection in 2014 with 2004.
PA infection was defined as one positive culture on an airway sample (either a sputum, throat swab or broncho-alveolar lavage sample). The modified Leed’s criteria was used to classify PA infection into never infected, free of infection (negative PA culture for > 12 months), intermittent infection ( positive PA culture for < 6months) and chronic infection ( positive PA culture for > 6 months) 17.
Patients were grouped into 3 age groups (0-5 years, 6-11 years, and 12-18 years) and the prevalence of PA infection has been compared in both years among the three different age groups. Additional data including the patients’ best FEV1 value performed over the year 2014, height, weight and body mass index (BMI) on the same day of best FEV1 were collected to examine for clinical differences between the four classifications of PA infection.
Statistical Analysis
Statistical analysis was performed using chi-squared testing for categorical variables and independent students t-tests for continuous variables (SPSS 20, IBM)
Results
Demographics of the patient population:
One hundred and sixty (160/166) patients in 2004 and 164/169 patients in 2014 were included in the final analysis. Eleven children were excluded from both cohorts (n= 6 in 2004 and n=5 in 2014) due to transfer to another service, missing data, death or being more than 18 years of age. The mean age was 9.35 ± 5.61 years in 2004 and 9.4 ± 5.27 years in 2014 (p-value =0.34), with range of 0-18 years. There was no significant statistical difference in the baseline characteristics between the two cohorts. See table 1 for the distribution of CF patients among the different age groups in 2004 in comparison to 2014. 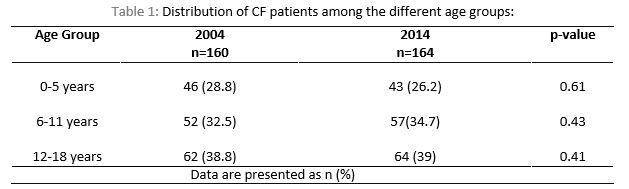
Prevalence of PA:
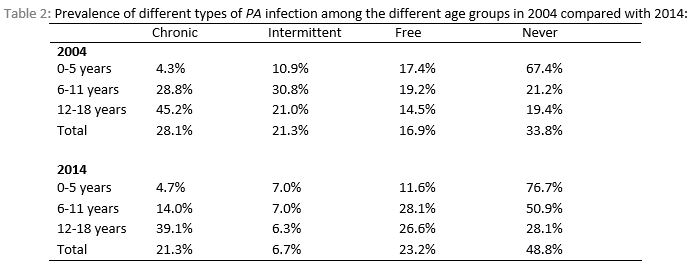
In 2004, out of 160 CF patients, 45 (28.1%) had chronic infection, 34 (21.2%) had intermittent infection, 27 (16.9%) were free of infection and 54 (33.8%) of children with CF were never infected with PA (Figure 1). In 2014, out of 164 children with CF, 35 (21.3%) had chronic infection, 11 (6.7%) had intermittent infection, 38 (23.2%) were free of infection for at least 12 months and 80 (48.8%) had no previous PA infection. There was no significant decline in chronic PA infection in 2014 compared with 2004 (p-value=0.2). However, there was a significant decline in intermittent infection and a significant increase in never infected group in 2014 compared with 2004 (P value < 0.001 and 0.007 respectively). There was no significant decline detected in the free from infection group between 2004 and 2014 (p-value=0.17). See figure 1. The likelihood of chronic infection increased with age in both cohorts in comparison to the never infected which decreased with increasing age, see Table 2.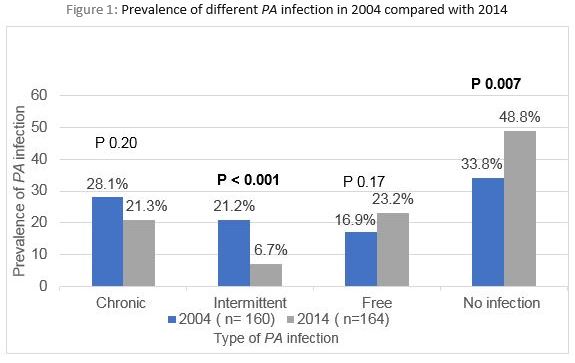
FEV1 characteristics:
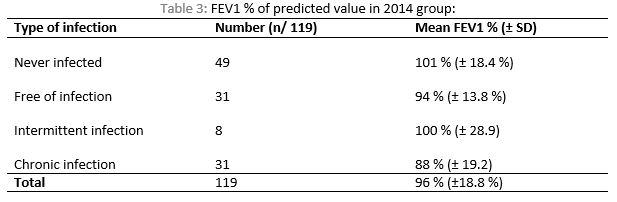
The mean FEV1% predicted value in 2014 for those above 6 years of age was 96% ±18.8%(range of 41 - 142 %). Among those who have chronic PA infection (n=31), only one patient had FEV1% predicted value < 60 %. The mean FEV1 among the different PA infection groups was: 101.4%, 94.4%, 100.4% and 88.2% for never infected group, Free of infection, intermittent infection and chronic infection respectively, see Table 3. The chronic PA infection group had lower FEV1% compared with other groups (P value=0.009). There was no significant difference in FEV1 among the remaining three groups.
Discussion
In our single center, retrospective, observational study, we have observed a significant decline in the prevalence of PA infection, and a change in infection pattern over a 10-year period.
In our study, the overall prevalence of PA infection was 28 % in 2014 (21.3% chronic and 6.7% intermittent infection). A recent Irish study examining PA infection rates in CF patients reported a slightly higher prevalence rate of 31% (19% chronic and 12% intermittent infection). However, this study included both adults and children between 4-69 years of age and the median age was 18.1 years16.
There are many classification systems used to classify PA infection in the literature which led to variability in reported prevalence of PA infection in children and adults with CF. Based on the recommendation by the European CF Society patient registry guidelines, we have used Leed’s criteria to define PA infection in this study.
The prevalence of chronic PA infection in our study was 21.3 % which is less compared with previously reported French prevalence of 56 %19, Swedish prevalence of 30.9 %20 and of Australian multi antibiotics resistant PA prevalence of 31%21. However, the prevalence in this study was more than the reported prevalence of chronic infection in children with CF in Denmark, which was 14.5%20. Of note, previously mentioned studies have used different criteria to classify PA infection, where they defined chronic infection as repeated positive bacterial cultures (≥3) during the last six months and/or 2 or more crossed immunoelectrophoresis (CIE) precipitating antibodies against PA. However, in a recent US study which used Leed’s criteria, the prevalence of chronic PA infection in the adolescent age group is 40 % which is similar to the calculated prevalence in our study for the adolescent age group (39.1%). We reported a lower prevalence in the younger age groups (4.7 % in those below 5 years of age and 14 % in 6-10 years of age) compared to US data (20 % in those below 5 years and 25 % in 6-10 years of age)14.
Our results showed an interestingly significant decline in the prevalence of intermittent PA infection and increase in the number included in the never infected group over the past ten years. A US study also reported a decline in overall PA infection prevalence in younger patient with CF (< 18 years of age) from 50.7 % in 1995 to 30.4 % in 201514. The reason for such decline in our center is likely multifactorial. Newborn screening for CF was introduced into Ireland in 2011 and it is likely that earlier diagnosis plays a significant role in the decline of chronic PA infection rates. Other changes to the CF service over this timeframe include increased number of CF multidisciplinary team members, improved PA eradication treatment options and a strict patient segregation policy during hospital attendances for all CF patients.
There are several limitations to consider with our study. We acknowledge that this is a single center study but given the fact that OLCHC is the largest tertiary paediatric CF center in Ireland, the above findings likely provide an estimate of the true overall prevalence of PA infection in children with CF in Ireland.
Acknowledgments
We would like to thank Abaigeal Jackson (Cystic fibrosis registry of Ireland) and Dr Catriona Logan for their valuable support in retrieving the needed data. Our appreciation extends to Lisa Martyn, Barbara Brudell and Mairead Ryan who contributed in completing the data collection. Preliminary results of the present work have been presented at Irish thoracic society meeting on 9th of November 2017.
Conflicts of Interest Statement
I declare that I have no conflict of interest.
Corresponding Author
Khoula Al Shidhani,
Our Lady’s Children’s Hospital,
Crumlin,
Dublin
Email: [email protected]
References
1- Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2012; 109(15):5809–14.
2- Birgitte Frederiksen, Christian Koch, Niels Høiby.Changing epidemiology of PsA infection in Danish cystic fibrosis patients. Pediatric pumonolgy, 1999.
3- Treggiari MM, Rosenfeld M, Retsch-Bogart G, Gibson R, Ramsey B. Approach to eradication of initial Pseudomonas aeruginosa infection in children with cystic fibrosis. Pediatr Pulmonol. 2007 Sep;42(9):751-6.
4- Nicole Mayer-Hamblett, Bonnie W. Ramsey,Hemantha D. Kulasekara, Daniel J. Wolter, Laura S. Houston, Christopher E. Pope, Bridget R. Kulasekara, Catherine R. Armbruster, Jane L. Burns, George Retsch-Bogart, Margaret Rosenfeld, Ronald L. Gibson, Samuel I. Miller, Umer Khan and Lucas R. Hoffman. Pseudomonas aeruginosa Phenotypes Associated with Eradication Failure in Children with Cystic Fibrosis. Clin Infect Dis. 2014 Sep 1; 59(5): 624–631.
5- Rosenfeld M, Ramsey BW, Gibson RL. Pseudomonas acquisition in young patients with cystic fibrosis: pathophysiology, diagnosis, and management. Curr Opin Pulm Med. 2003 Nov;9(6):492-7.
6- Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–9. 139.e1.
7- Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100.
8- Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr. 2001;138(5):699–704.
9- Zarmina Ehsan and John P Clancy. Management of Pseudomonas aeruginosa infection in cystic fibrosis patients using inhaled antibiotics with a focus on nebulized liposomal amikacin. Future Microbiol. 2015 Dec; 10(12): 1901–1912. doi: 10.2217/fmb.15.117.
10- Catriona Logan, Adele Habington, Gráinne Lennon, Frank Cronin, Niamh O'Sullivan. Evaluation of the efficacy of real-time polymerase chain reaction for the routine early detection of Pseudomonas aeruginosa in cystic fibrosis sputum and throat swab specimens. Diagnostic Microbiology and Infectious Disease 68 (2010) 358–365
11- Giovanni Taccetti, Elisa Bianchini, Lisa Cariani, Roberto Buzzetti, Diana Costantini, Francesca Trevisan, Lucia Zavataro, Silvia Campan. Early antibiotic treatment for Pseudomonas aeruginosa eradication in patients with cystic fibrosis: a randomised multicentre study comparing two different protocols. Thorax 2012;67:853–859. doi:10.1136/thoraxjnl-2011-200832.
12- Taccetti G, Campana S, Festini F, Mascherini M, Döring G. Early eradication therapy against pseudomonas aeruginosa in cystic fibrosis patients European Respiratory Journal 2005 26: 458-461.
13- Felix Ratjen, Anne Munck, Pearl Kho, Gerhild Angyalosi. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial.Thorax, 2009: 65:286-291.
14- Annual Data Report 2015. Cystic Fibrosis Foundation Patient Registry
15- Anjali Y. Bhagirath, Yanqi Li, Deepti Somayajula, Maryam Dadashi, Sara Badr and Kangmin Duan. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulmonary Medicine (2016) 16:174. DOI 10.1186/s12890-016-0339-5.
16- Emma Reece, Ricardo Segurado, Abaigeal Jackson, Siobhán McClean, Julie Renwick and Peter Greally. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: an Irish registry analysis. BMC Pulmonary Medicine (2017) 17:70. DOI 10.1186/s12890-017-0416-4.
17- M. Proesmans, W. Balinska.Miskiewicz, L. Dupont, X. Bossuyt, J. Verhaegen, N. Høiby, K. de Boeck. Evaluating the “Leeds criteria” for Pseudomonas aeruginosa infection in a cystic fibrosis centre. European Respiratory Journal 2006 27: 937-943
18- Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibrosis 2003;2(1):29-34.
19- John J. LiPuma. The Changing Microbial Epidemiology in Cystic Fibrosis. Clin Microbiol Rev. 2010 Apr; 23(2): 299–323.
20- Knudsen PK, Olesen HV, Høiby N, Johannesson M, Karpati F, Laerum BN, Meyer P, Pressler T, Lindblad A; Scandinavian CF Study Consortium (SCFSC). Differences in prevalence and treatment of Pseudomonas aeruginosa in cystic fibrosis centres in Denmark, Norway and Sweden. J Cyst Fibros. 2009 Mar;8(2):135-42. DOIi: 10.1016/j.jcf.2008.11.001. Epub 2009 Jan 20.
21- Smith DJ, Ramsay KA, Yerkovich ST, Reid DW, Wainwright CE, Grimwood K, Bell SC and Kidd TJ. Pseudomonas aeruginosa antibiotic resistance in Australian cystic fibrosis centres. J Cyst Fibros. 2009 Mar;8(2):135-42. doi: 10.1016/j.jcf.2008.11.001. Epub 2009 Jan 20.
Issue: Ir Med J; Vol 112; No. 6; P946
