The use of cold coagulation for the treatment of cervical intraepithelial neoplasia
Wyse A1, Seah W A1, O’Neill J1. Byrne P1 2.
1Colposcopy Department, Rotunda Hospital, Dublin 1, Ireland
2Department of Obstetrics and Gynaecology, Royal College of Surgeons, 123 St. Stephens Green, Dublin 2, Ireland.
Abstract
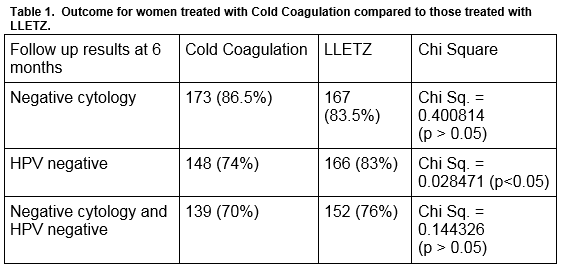
In 2015, Cold Coagulation was introduced as a treatment for cervical intraepithelial neoplasia (CIN) at our colposcopy clinic. We reviewed the 6-month follow up data of the first 200 women who underwent Cold Coagulation using cytology and HPV status as tests of cure (TOC). A random sample of 200 patients treated by Large Loop Excision of the Transformation Zone (LLETZ) during the same period was used to compare treatment outcome. Six months following treatment,173 (86.5%) of the women treated by CC and 167 (83.5%) treated by LLETZ had negative cytology. (x2= P>0.05). 148 (74%) treated by Cold Coagulation and 166 (83%) treated by LLETZ were HPV negative (x2= P<0.05). One hundred and thirty-nine (70%) women treated by Cold Coagulation and 152 (76%) treated with LLETZ had normal cytology and were HPV negative. This audit of our initial experience supports the observation that Cold Coagulation is as effective as LLETZ in the management of CIN when cervical cytology is used as a test of cure.
Introduction
Cervical Check, Ireland’s National Cervical Screening Programme was set up in September 20081. The programme provides free cervical smears to all women aged between 25 and 60 years to detect precancerous changes in the cervix. If abnormalities are detected on cytology or for clinical reasons, patients are referred for colposcopy. The colposcopy service identifies and treats patients with precancerous disease to prevent progression to cervical cancer2. To date, Large Loop Excision of the Transformation Zone (LLETZ) has been the main treatment provided1. Between 1.9.2013 to 31.8.2014 6,725 treatments were performed in Ireland1. Of these 5,674 (84.4%) were LLETZ procedures1. However, there is increasing evidence that LLETZ for treatment of cervical intraepithelial neoplasia (CIN) is associated with preterm delivery and may be associated with increased perinatal mortality3,4,5.
Cold Coagulation is an ablative technique that was developed by Kurt Semm in the 1960’s. Prior to treatment, a biopsy is taken to confirm the presence of CIN and exclude invasion. The Semm cold coagulator is heated to 100 °C, and applied to the transformation zone of the cervix for 30 seconds6, 7. Two, three, or four applications may be used depending on the size of the transformation zone. A recent meta- analysis looking at the efficacy of Cold Coagulation has found it to be as effective as LLETZ for treating CIN, with cure rates of up to 96%, and having the added benefit of no documented negative impact on fertility and subsequent pregnancies6. Until recently, eradication of CIN was assessed by cervical cytology, six and eighteen months following treatment8. In 2014, the National Cervical Screening Programme introduced an additional test of cure which assesses the presence of high risk Human Papilloma Virus (HPV) subtypes8. In the light of recent publications highlighting the adverse effects of LLETZ on pregnancy outcome, we introduced Cold Coagulation as a treatment option for some of our patients. This study assesses our initial experience with this treatment modality.
Methods
This study was undertaken on the first 200 women who underwent cold coagulation and for whom there was six months follow up data. Data was collected prospectively for patients treated between September 2014 and September 2015. As per the National Cancer Screening Service Standard Guidelines all patients were required to have a colposcopically directed punch biopsy8. Patients with persistent low grade disease (CIN 1) or higher grade disease (CIN 2 or 3) were invited back to the clinic and were given the option of having a cold coagulation treatment if they fulfilled the criteria for treatment using this technique8. Patients were not considered suitable for cold coagulation if they were pregnant, if they had a previous ablative or excisional treatment, if the entire transformation zone was not visible, if there was suspicion of endocervical involvement, if there was evidence or suspicion of glandular or invasive disease or a discrepancy between the cytology and biopsy. At the clinic the procedure was explained to the patients and written consent was obtained. Patient data was recorded in the Mediscan colposcopy database and the APEC pathology database. A random sample of 200 patients who underwent a LLETZ procedure during the study period and who had follow up data available at 6 months was used to compare treatment success. All patients had a smear and HPV test done in the colposcopy clinic six months following treatment. Success was defined as negative cytology or a negative HPV test (for high risk sub-types HPV-16 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68). The data was analysed in Excel. Ethical approval was obtained from the Clinical Audit department of the Rotunda Hospital.
Results
Data on the first 200 patients who were treated with cold coagulation are presented. The mean age was 31 years (SD = 6.37 years). One hundred and fifteen (57.5%) were nulliparous. Pre-treatment biopsy histology was as follows: CIN 1: 7 (3.5%), CIN 2: 146 (73%), CIN 3: 47 (23.5%).
Similar outcome data was analyzed for a random sample of 200 patients who had been treated with LLETZ during the same period. The mean age of this group was 36 years (SD = 8.29), 51 (25%) were nulliparous. Pre–treatment referral smear cytology for the LLETZ group was as follows : AGUS : 11 (5.5%), ASC-H :19 (9.5%), GIN :1 (0.5%), LSIL :43 (21.5%), HSIL :113 (56.5%). Thirteen (6.5%) were referred for clinical reasons.
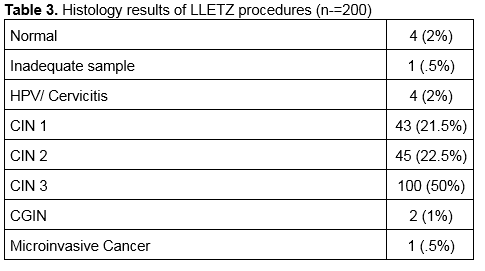
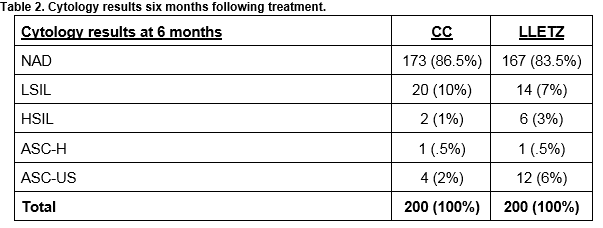
Patients were followed up at 6 months with a cervical smear and HPV test. The results are shown in Table 1. The cytology results at 6 months for women treated with Cold Coagulation and for those treated with LLETZ are shown in Table 2. The histology results for the 200 women treated with LLETZ are shown in Table 3. None of the women treated with cold coagulation developed any serious complications. One woman was reviewed in the clinic 7 days following treatment complaining of a heavy vaginal discharge. Microbiological culture failed to grow any pathogens in this case.
Discussion
This is an audit of our initial experience with cold coagulation, which was introduced in 2015 as a treatment option for patients with cervical intra-epithelial neoplasia. Subject to strict criteria, it is offered as a choice for nulliparous women or for those whose families are incomplete. We decided to introduce Cold Coagulation as a treatment for our patients because the current gold standard treatment, LLETZ, has been linked with long term obstetrical complications such a preterm labour and miscarriage3,4. Published studies to date have used cytology alone as a test of cure to measure treatment success. HPV testing was introduced in 2014 by the National Cancer Screening Service as a test of cure following treatment of CIN. IN view of this, we reviewed both cytology and HPV status as tests of cure8. Despite our relative inexperience with this treatment modality, our results have been reassuring. Using cytology as a test of cure, cold coagulation was successful in 173 (86.5%) women (Table1). This compares favourably with other national studies. A recent study in University Hospital Limerick by McCarthy et al, included 93 patient who were treated by cold coagulation and reported treatment success of 79.9% for patients with CIN1 and 81.1% for patients with CIN2/39. A study in Northern Ireland reviewed 725 patients who underwent cold coagulation and reported negative cytology in 632 (87.1%) cases10. However when compared with international data, our cure rate is lower. A study in the UK by Parry-Smith et al which looked at 557 patients who were treated by cold coagulation over a ten-year period recorded treatment success rates of 95.7%11. Similarly, a study done by Duncan in the early 1990’s looked at 1,628 women with CIN3 treated by cold coagulation, and found that disease was eradicated in 95% of cases when reviewed at one year7.
Our study reports cure rates at 6 months following treatment. Most of the published reports have longer periods of follow up. This is one factor that may be responsible for our lower cure rates. We were anxious to determine if cold coagulation was as effective as LLETZ, so we audited our cure rates at six months rather than waiting for 18 months.When cytology was assessed six months following treatment, there was no significant difference in cure rates for women treated with cold coagulation compared to those treated with LLETZ. Using cytology alone as a test of cure six months following treatment, 167 (83.5%) women treated with LLETZ had a normal cervical smear compared to 173 (86.5%) treated with cold coagulation. We are mindful that the two groups are different in many aspects including age, parity and disease distribution and indeed many patients considered for LLETZ would be deemed unsuitable for ablative treatment. However, prior to the introduction of cold coagulation, this group of women would have been offered a LLETZ treatment. Cold coagulation is primarily offered to a specific group of women who are younger and nulliparous or with incomplete families. However. the comparison is presented to allow us to assess the relative success of cold coagulation in our unit.
To date, very little data has been published using HPV status as a test of cure following cold coagulation. Reviewing HPV status at 6 months following treatment 148 (74%) of those treated by cold coagulation were HPV negative. This compares less favourably to the group of patients treated by LLETZ with 166 (83%) cases HPV negative at 6 months following treatment. The difference is statistically significant. While studies have shown that HPV testing is helpful as an adjunct to cytology for its high negative predictive value,12,13,14 its validity as a test of cure indicator is less reliable due to the lack of available data on the natural history of the HPV infection. Kim et al tracked the clearance of HPV infection after conization and noted that persistent HPV infections were detected in 45.6%, 14.3%, 6.3%, 2.2%, 1.5% and 1.1% of patients at 3, 6, 9, 12, 18 and 24 months after loop excision15. This leads us to question if using HPV as a test of cure at 6 months following treatment is too early to accurately evaluate the effectiveness of our treatment. Indeed, we now see many women in our unit who are cytology negative at 6 months following treatment, but who are still HPV positive. Prior to the introduction of HPV testing, such women would have been asked to return 12 months later for a second cervical smear. It is now recommended practice that women who are cytology negative but HPV positive six months following treatment should be reassessed at the colposcopy clinic. This practice has led to an increased the number of women who need a repeat colposcopy following treatment.
Previous studies have shown that cold coagulation is well tolerated by women and has minimal side effects and no documented long term impact on fertility6,9. We found cold coagulation to be a very safe procedure with no significant complications. One of the main reasons for a renewed interest in cold coagulation relates to the potential pregnancy morbidity that may be associated with LLETZ. The volume of cervical tissue removed is important. Studies have shown that the deeper the excision (>10 mm) or greater amount of tissue removed, the higher the risk of premature delivery in subsequent pregnancies5. During cold coagulation, on average 4-7mm of the cervix is ablated6. It is presumed that cold coagulation is likely to be associated fewer pregnancy related complications than excisions techniques such as LLETZ although this data has not been published. However, morbidity would be expected to be low based on a meta-analysis of perinatal and obstetric outcomes that included other forms of ablation5. There is an urgent need for a randomised controlled trial comparing the safety of LLETZ and Cold Coagulation, particularly in relation to pregnancy morbidity.
Correspondence:
Prof Paul Byrne MD FRCOG FRCPI, Colposcopy Clinic, Rotunda Hospital, Dublin 1
Email: [email protected]
Conflict of Interest
The authors have no conflicts of interest.
References
1. Irish Cervical Screening Programme Cervicalcheck. Cervicalcheck Programme Report 2013- 2014. Available at: http://www.cervicalcheck.ie/_fileupload/CervicalCheck%20Programme%20Report%202013-2014%20(web).pdf (Accessed: 8th July 2016).
2. Irish Cervical Screening Programme Cervicalcheck. Referral to Colposcopy. Available at: http://www.cervicalcheck.ie/colposcopy/referral-to-colposcopy.5658.html (Accessed: 8th July 2016).
3. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial of early invasive cervical lesions: systematic review and meta analysis. Lancet 2006;367:489–98.
4. Khalid S, Dimitriou E, Conroy R, Paraskevaidis E, Kyrgiou M, Harrity C, Arbyn M, Prendiville W. The thickness and volume of LLETZ specimens can predict the relative risk of pregnancy related morbidity. BJOG 2012 ;119 : 685-691.
5. Arbyn M, Kyrgiou M, Simoens C, O Raifu A, Koliopoulos G, Martin-Hirsch P, Prendiville W, Paraskevaidis E. Perinatal morbidty and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ 2008; 337:a1284
6. Dolman L, Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the efficacy of cold coagulation as a treatment method for cervical intraepithelial neoplasia. BJOG 2014; 121: 929-942.
7. Gordon HK, Duncan ID. Effective destruction of cervical intraepithelial neoplasia (CIN) 3 at 100 degrees C using the Semm cold coagulator: 14 years experience. BJOG 1991; 98: 14-20.
8. National Cancer Screening Service. Guidelines for Quality Assurance in Cervical Screening. CervicalCheck. Report number: 2nd Edition, 2014. Available from : http://www.cervicalcheck.ie/_fileupload/Publications/Final.pdf
9. C.M McCarthy, M. Ramphul, M. Madden, K. Hickey. The use and success of cold coagulation for the treatment of high grade squamous cervical intra-epithelial neoplasia : a retrospective review. EJOG 203 (2016) ; 225-228.
10. Zawislak A, Price JH, McClelland HR, Storey RGN, Caughley L. Efficant of cervical intraepithelial neoplasia (CIN) treatment by cold coagulation. Ulster Med J. 2003 May ; 72(1) : 10-15.
11. Parry-Smith W, Underwood M, De Belis-Ayres S, Bangs L, Redman C, Panikkar J. Success rate of cold coagulation for the treatment of cervical intraepithelial neoplasia : A retrospective Analysis of a Series of Cases. Journal of Lower Genital Tract Disease Jan 2015 ; Vol 19, Issue 1: 17-21.
12. Kitchener HC, Walker PG, Nelson L, Hadwin R, Patrick J, Anthony GB, Sargent A, Wood J, Moore C, Cruickshank ME. HPV testing as an adjunct to cytology in the follow up of women treated for cervical intraepithelial neoplasia. BJOG 2008 ; 115 : 1001-7.
13. Mesher D, Szarewski A, Cadman L, Cubie H, Kitchener H, Luesley D, MEnon U, Hulman G, Desai M, Ho L, Terry G, Williams A, Sasieni P, Cuzicki J. Long-term follow up of cervical disease in women screen by cytology and HPV testing : results from the HART study. Br J Cancer ; 27 : 1405-10.
14. Paraskevaidis E, Arbyn M, Sotiriadis A, Diakomanolis E, Martin-Hirsch P, Koliopoulos G, Makrydimas G, Tofoski J, Roukos DH. The role of HPV DNA testing in the follow up period after treatment for CIN: a systematic review of the literature. Cancer Treat Rev ; 30 : 205-11.
15. Kim YT, Lee JM, Hur SY, Cho CH, Kim YT, Kim SC, Kang SB. Clearance of human papillomavirus infection after successful conization in patients with cervical intraepithelial neoplasia. Int J Cancer 2010 ; Apr 15 : 126 (8) :1903-9
(P565)