Trends in surgical mortality following colorectal resection between 2002 and 2012: A single-centre, retrospective analysis.
I Stephens, C Stuart, R Stephens, P McCormick, J Larkin, B Mehigan
GEMS Directorate, St. James’s Hospital, Dublin 8
Abstract
Surgical mortality is a commonly-used measurement of surgical risk. It is imperative that patients receive accurate, up-to-date information regarding operative risk. To date, studies investigating temporal changes in surgical mortality following colorectal resection in Ireland have been limited. This retrospective study investigates such trends in one of the eight centres for symptomatic and screen-detected colorectal cancers in Ireland, across an 11-year period. A steady decline in surgical mortality was found across this time, showing a significant difference in rates before and after centralisation of rectal cancer care and the advent of colorectal surgery as a surgical specialisation (5.2%, 1.52%). This has important implications for the organisation of colorectal cancer care in Ireland.
Introduction
Colorectal cancer accounts for 13.4% of all cancer in the Republic of Ireland, and is the third most common cause of death resulting from cancer1. Since 2007, rectal cancer care has been centralised to eight cancer centres. Furthermore, screen-detected colon cancer can only be operated on in these centres. These measures have coincided with the establishment of colorectal surgery as a surgical speciality, and an increased emphasis on multidisciplinary team provision of care. Preliminary data suggests that this refined approach to treatment has modernised rectal cancer management across the country; improved quality of surgical resection, and a reduction in the administration of post-operative radiotherapy was seen between 2007 and 20102. However, the relationship between surgeon and hospital caseload to overall quality of care, as determined by a wide range of surgical, pathological, preoperative and post-operative parameters is inconsistent, and does not appear to be a simple linear relationship3. Though this may be the case, surgical outcomes remain an informative data source for audit and analysis.
Of note, no investigations of temporal trends in Irish surgical mortality for colon and rectal resection have been completed to date. Previous studies have reported on rates for single years, mean rate across several years, or comparisons between two given years2,4. While this information provides us with a snapshot view of the current state of affairs, it does not provide definitive answers to the fundamental question: have the changes implemented in the organisation of care resulted in less patients dying in the perioperative period? Statistical validation of the new architecture for care is required across the country.
Post-operative or surgical mortality, defined as death within 30-days of surgery, is a commonly-used measurement of surgical risk5. Accurate, up-to-date data is of vital importance both to the clinician and the patient in guiding the patient counselling and consenting process. Numerous predictive models of post-operative mortality following colorectal resection have been developed, including the CR-POSSUM, ACPBGI, and revised ACPBGI models6,7,8. These models use patient demographics and physiological variables to estimate the risk of surgical mortality for an individual patient. Such models, however, cannot account for temporal changes, nor local practices and expertise which may influence the absolute surgical mortality and morbidity for any given centre.
The Association of Coloproctology of Great Britain and Ireland (ACPBGI) report on operative mortality at the time showed an unexpectedly high 30-day mortality rate of 7.5% across a 12 month period between 1999 and 20016. A more recent analysis of the Revised ACPGBI model reports a mean operative mortality of 4% from 1997-20078. Data from Ireland suggests a considerably a lower rate for rectal cancer in recent years; however, no study has looked at colorectal resection as a whole. Two Irish studies of rectal resection completed since the centralisation of care report surgical mortality rates of 1.7% and 1.5%, and 1.1% for 2007 and 2010 respectively2,4. International studies have further demonstrated a reduction in post-operative 30-day mortality following colorectal resection in recent decades. Analysis of Canadian veterans who underwent colorectal resection found a decline in surgical mortality rates from 4.7% during 1987-1988 to 3.9% in 1998-2000, and date of surgical resection was identified as an independent risk factor for post-operative mortality9. Similarly, an investigation of an Italian colorectal registry highlighted a downward trend in post-operative mortality across 1984-2004, with a reduction from 7-11% to 3-6%10. We hypothesize that a similar trend in surgical mortality should be seen for colorectal resection in Irish specialist units throughout the 2000s.
Methods
Data was extracted from the St. James’s Hospital Colorectal database11. The database has been prospectively maintained since 2001, and includes data on 3,703 patients. Approximately 700 fields are completed for each patient to the highest possible degree by a dedicated colorectal data-manager, using patient charts and records. Fields include patient demographics, histology, surgery, chemotherapy, radiotherapy, and follow up. Historical patient histology predating 2001, patients referred for multidisciplinary discussion but who had their primary care elsewhere, and patients referred from other hospital to non-surgical services constitute a significant portion of the entries contained in the database.
To isolate the desired cohort of patients the database was queried by year, colorectal cancer diagnosis, operating hospital and operation type. Patients who underwent colorectal resection at St. James’s Hospital between December 31st, 2001 and January 1st, 2013 were selected for inclusion. Colorectal resections performed included right hemicolectomy, left hemicolectomy, sigmoidectomy, subtotal colectomy, total abdominal colectomy, transverse colectomy, proctocolectomy, panproctocolectomy, anterior resection, abdominoperineal resection, or Hartman’s procedure. At the time of analysis, data for 2013-2015 had not yet been validated and was hence excluded from this study. Age at registration, date of operation, date of death, date of diagnosis, TMN staging, metastatic status, level of urgency, tumour site, synchronous tumours, local complications, intent of surgery, surgical complications, cause of death, and ACPGBI and CR-POSSUM % mortality were extracted from the database. Nine hundred and eighty-seven entries were initially identified. In this group, there were 28 duplicate patient entries due to the presence of synchronous tumours at time of diagnosis. These repeats were removed resulting in a cohort of 959 unique patients for analysis. Three hundred and sixty-five of these patients underwent resection for rectal cancers, the remainder had colonic tumours. The overall patient population demographics and characteristics were tabulated and examined.
Date of death and date of operation were compared for all 959 patients. Patients who died within 30 days of their operation were considered surgical mortalities. The rate of surgical mortality was calculated for each year and compared across the 11-year period. The population demographics of the surgical mortality subgroup were tabulated and examined. Statistical analysis was carried out using R version 3.2.312, and GraphPad Prism 6 for Windows13. Linear regression analysis was used to evaluate trends in mortality across the time period. G*Power14 was used to estimate the sample size required for a prospective randomised control trial.
Results
Surgical Mortality
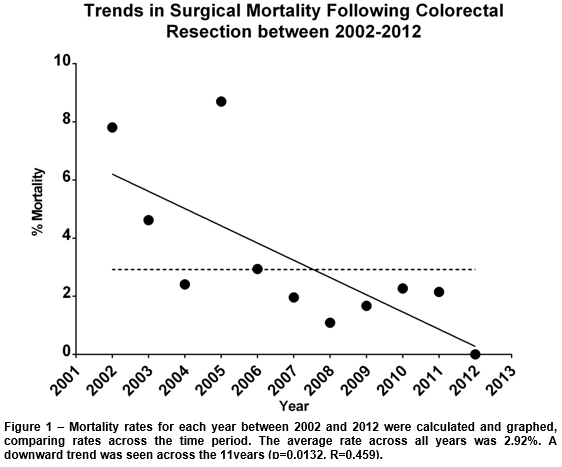
There was a total of 28 surgical mortalities across the 11-year period, with an average rate of 2.92%. The annual rates ranged from 0-8.7%, with the highest being 2005 and the lowest 2012 (Table 1). Overall, a significant downward trend was seen across these years (p=0.0132, R2=0.459) (Figure 1).
Patient Demographics
The gender profile between the two groups was comparable; there were 58.1% males in the total patient population, and 57% in the surgical mortality subgroup. The mean age (81±11 vs 68±12) was higher in the surgical mortality group, and only two patients were under the age of 70. Furthermore, 64.3% of the surgical mortality group were over 80, compared to 19.1% of the overall population. A higher relative proportion of emergency and palliative surgeries was seen in the surgical mortality group (25% and 28.6%) when compared to the overall population (5.4% and 8.7%). A higher percent of surgical mortality patients had metastatic disease at presentation (28.57% compared to 13%) (Table 2), as well as a higher incidence of local cancer complications (35.7% compared to 17.8%). 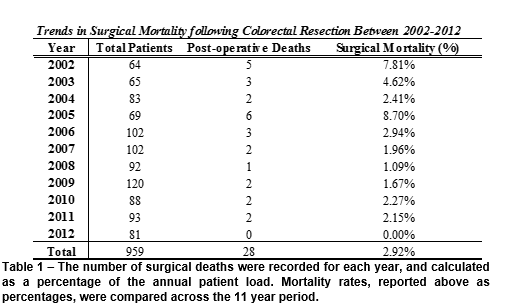
The average CR-POSSUM and ACPBGI percentage mortality were higher in the surgical mortality group (26.02% and 17.96%) than in the total population (7.7% and 5.4%)(Table 2), and all but one surgical mortality patient had one or more recorded post-operative complications. The most common post-operative complications were sepsis (10/28), organ failure (9/28), myocardial infarction (4/28), haemorrhage (2/28), deep vein thrombosis (2/28) and anastomotic leak (2/28).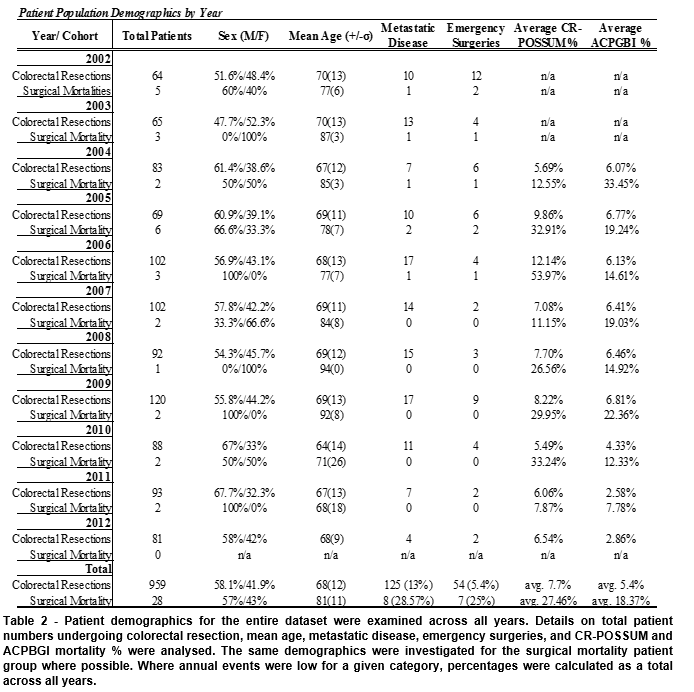
Discussion
These downward trends in surgical mortality following colorectal resection demonstrate a statistically and clinically significant improvement in operative mortality between 2002 and 2012. Multiple factors may be hypothesised to be behind this improvement. These include the advent of specialist colorectal surgeons, improvement in cancer staging, intensive care medicine and a team based care, whereby all patients are looked after by more than one consultant colorectal surgeon. Any or all of these factors may have contributed, though it is impossible to establish causality in a retrospective study of this nature. However, the magnitude of the improvements seen should not be overlooked. They are clinically significant and are of the same order as the population survival gain achieved by giving node negative patients adjuvant chemotherapy15. A prospective randomised controlled trial to look at such changes in mortality would require at least 400 patients to be adequately powered and is unlikely to ever be undertaken.
Prior to 2007, average mortality was 5.2% (range 2.41-8.7%). Thereafter, this figure dropped to 1.52% (range 0-2.27%), a greater than 3-fold reduction. The surgical mortality group suggests associations with well recognised colorectal surgery risk factors, including advanced age16, 17 and higher average CR-POSSUM and ACPBGI scores18,19. Multiple studies have shown that specialisation produces better surgical outcomes20-22. Lower anastomotic leak rates, higher local recurrence free survial20, lower rates of permanent stomas21 and lower peri-operative mortality for elective surgery22 have all been highlighted as benefits of specialist care.
Of note, resection for rectal cancer accounted for 38.1% of the overall caseload, but just 20.3% of the surgical mortalities. Of particular interest, the composition of the overall caseload changed after 2007. Prior to 2007, rectal cancer accounted for 35.5% (28.1-43.4%) of all cases, and 40.2% (29.4-48.2%) thereafter. In the same timeframe, perioperative mortality following rectal resection decreased by 4.8-fold from 4.77%, to 0.98% following a similar downwards trend (R2=0.49, p=0.036) to that seen for colorectal resection as a whole. Across this time, the average number of colorectal resections carried out in the unit increased from 76.6 (64-102) to 96 (81-120), a 25% increase. It is worth noting that prior to 2007, 8.4% of the all surgeries performed were classified as emergencies, when compared to just 3.8% thereafter. There was also a reduction in surgical mortality for this subgroup, falling from 21% (7/32) to 0% (0/22). The significant decreases in the number of emergency surgeries carried out may be attributable to the increased use of colonic stenting used both as a bridge to surgery, or as an alterative to emergency palliative operations for colonic obstruction in incurable disease. We have have published our favourable results of colonic stenting elsewhere23. Though the data is promising, the scarcity of emergency surgeries in the latter years makes it difficult to comment with confidence on mortality trends within this group as a subpopulation.
This dataset has captured a time period which encompasses major alterations in the delivery of care for colorectal cancer. It has highlighted one of the positive effects that has come about as a result. The Irish healthcare system has continued to develop the manner in which colorectal cancer is approached. Most recently, in 2014 the National BowelScreen program was introduced. This has the potential to greatly alter the population dynamics of patients undergoing colorectal resection, and may have a knock-on effect on key performance indicators for resection. It is pertinent that analysis into patient demographics and surgical mortality post implementation of this screening program occurs in the coming years. Colon cancer was not spefically centralised under the national cancer control programe yet screen detected colon cancer can only be operated on in one of the eight cancer centres. With emergency surgery and advanced age being the highest risks of surgical mortality it seems anomalous that the lowest risk screen detected patients can only be operated on in a cancer centre whilst the elderly high risk emergency presentation can be operated on in any of our acute or private hospitals. We suggest that an Irish national audit of colorectal cancer mortality is urgently needed to ascertain if these improvements in surgical mortality are seen nationally and if not seen nationally to ascertain why not.
Conflict of Interest
There are no conflicts of interest.
Correspondence:
Ian Stephens, GEMS Directorate, St. James’s Hospital, Dublin 8
Email: [email protected]
References
1. Comber H DD, Gavin AT. Cancer in Ireland 1994-2004: A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland; 2009.
2. Burke JP, Coffey JC, Boyle E, Keane F, McNamara DA. Early outcomes for rectal cancer surgery in the republic of ireland following a national centralization program. Ann Surg Oncol. 2013;20(11):3414-21.
3. Comber H, Sharp L, Timmons A, Keane FB. Quality of rectal cancer surgery and its relationship to surgeon and hospital caseload: a population-based study. Colorectal Dis. 2012;14(10):e692-700.
4. Boyle E, Timmons A, Al-Akash M, Kennedy AM, O'Grady H, Hill AD, Comber H, Keane FB. The management of rectal cancer in Ireland in 2007--room for improvement? Surgeon. 2011;9(4):179-86.
5. Russell EM, Bruce J, Krukowski ZH. Systematic review of the quality of surgical mortality monitoring. Br J Surg. 2003;90(5):527-32.
6. Tekkis PP, Poloniecki JD, Thompson MR, Stamatakis JD. Operative mortality in colorectal cancer: prospective national study. BMJ. 2003;327(7425):1196-201.
7. Tekkis PP, Prytherch DR, Kocher HM, Senapati A, Poloniecki JD, Stamatakis JD, Windsor AC. Development of a dedicated risk-adjustment scoring system for colorectal surgery (colorectal POSSUM). Br J Surg. 2004;91(9):1174-82.
8. Richards CH, Leitch EF, Anderson JH, McKee RF, McMillan DC, Horgan PG. The revised ACPGBI model is a simple and accurate predictor of operative mortality after potentially curative resection of colorectal cancer. Ann Surg Oncol. 2011;18(13):3680-5.
9. Davila JA, Rabeneck L, Berger DH, El-Serag HB. Postoperative 30-day mortality following surgical resection for colorectal cancer in veterans: changes in the right direction. Dig Dis Sci. 2005;50(9):1722-8.
10. de Leon MP, Pezzi A, Benatti P, Manenti A, Rossi G, di Gregorio C, Roncucci L. Survival, surgical management and perioperative mortality of colorectal cancer in the 21-year experience of a specialised registry. Int J Colorectal Dis. 2009;24(7):777-88.
11. Cancer Audit Programme Team SJsH. Ten year Cancer Audit Report St. James’s Hospital. St. James Hospital, Ireland; 2003-2012.
12. R Development Core Team. R: A language and environment for statistical computing. 3.2.3 ed. Vienna, Austria: R Foundation for Statistical Computing; 2015.
13. Software G. Graphpad Prism version 6.00 for Windows. La Jolla California USA2015.
14. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-91.
15. Group QC. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. The Lancet. 2007;370 (9604 ):2020 - 9.
16. Gomes A, Rocha R, Marinho R, Sousa M, Pignatelli N, Carneiro C, et al. Colorectal surgical mortality and morbidity in elderly patients: comparison of POSSUM, P-POSSUM, CR-POSSUM, and CR-BHOM. Int J Colorectal Dis. 2015;30(2):173-9.
17. Tan KY, Kawamura Y, Mizokami K, Sasaki J, Tsujinaka S, Maeda T, Konishi F. Colorectal surgery in octogenarian patients--outcomes and predictors of morbidity. Int J Colorectal Dis. 2009;24(2):185-9.
18. Ozkan O, Guner A, Kaya U, Kece C, Reis E, Kesici S, et al. Evaluation of CR-POSSUM, original ACPGBI and new ACPGBI scoring systems for colorectal cancer surgery. Chirurgia (Bucur). 2014;109(6):800-5.
19. Teeuwen PH, Bremers AJ, Groenewoud JM, van Laarhoven CJ, Bleichrodt RP. Predictive value of POSSUM and ACPGBI scoring in mortality and morbidity of colorectal resection: a case-control study. J Gastrointest Surg. 2011;15(2):294-303.
20. Smith JA, King PM, Lane RH, Thompson MR. Evidence of the effect of 'specialization' on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg. 2003;90(5):583-92.
21. Shankar PJ, Achuthan R, Haray PN. Colorectal subspecialization in a DGH. The way forward! Colorectal Dis. 2001;3(6):396-401.
22. Borowski DW, Kelly SB, Bradburn DM, Wilson RG, Gunn A, Ratcliffe AA, Northern Region Colorectal Cancer Audit Group. Impact of surgeon volume and specialization on short-term outcomes in colorectal cancer surgery. Br J Surg. 2007;94(7):880-9.
23. Larkin JO, Moriarity AR, Cooke F, McCormick PH, Mehigan BJ. Self-expanding metal stent insertion by colorectal surgeons in the management of obstructing colorectal cancers: a 6-year experience. Tech Coloproctol. 2014;18(5):453-8.
(p578)